Abstract
Previous studies have demonstrated that poultry house workers are exposed to very high levels of organic dust and consequently have an increased prevalence of adverse respiratory symptoms. However, the influence of the age of broilers on bioaerosol concentrations has not been investigated. To evaluate the evolution of bioaerosol concentration during the fattening period, bioaerosol parameters (inhalable dust, endotoxin and bacteria) were measured in 12 poultry confinement buildings in Switzerland, at three different stages of the birds’ growth; samples of air taken from within the breathing zones of individual poultry house employees as they caught the chickens ready to be transported for slaughter were also analysed. Quantitative polymerase chain reaction (Q-PCR) was used to assess the quantity of total airborne bacteria and total airborne Staphylococcus species. Bioaerosol levels increased significantly during the fattening period of the chickens. During the task of catching mature birds, the mean inhalable dust concentration for a worker was 26 ± 1.9 mg m−3 and endotoxin concentration was 6198 ± 2.3 EU m−3 air, >6-fold higher than the Swiss occupational recommended value (1000 EU m−3). The mean exposure level of bird catchers to total bacteria and Staphylococcus species measured by Q-PCR is also very high, respectively, reaching values of 53 (±2.6) × 107 cells m−3 air and 62 (±1.9) × 106 m−3 air. It was concluded that in the absence of wearing protective breathing apparatus, chicken catchers in Switzerland risk exposure beyond recommended limits for all measured bioaerosol parameters. Moreover, the use of Q-PCR to estimate total and specific numbers of airborne bacteria is a promising tool for evaluating any modifications intended to improve the safety of current working practices.
Keywords: airborne bacteria, bioaerosols, endotoxin, occupational health, poultry farm, Staphylococcus xylosus
INTRODUCTION
Airborne and settled particulate material of biological origin is referred to, in the field of occupational health, as organic dust. In poultry houses, large-scale production has led to increased bird densities within buildings (Sauter et al., 1981). Such high densities of animals kept within confined space are a source of human health problems related to organic dust exposure (Thelin et al., 1984; Morris et al., 1991; Donham et al., 2000; Rylander and Carvalheiro, 2006). This organic dust is composed both of non-viable particles, generated by such things as faeces, litter, feed, feather formation (which produces a high quantity of allergen dandruff) and of viable particulate matter (also called bioaerosols). Bioaerosols are comprised of airborne bacteria, fungi, viruses and their by-products, endotoxin and mycotoxin. Exposure to bioaerosols in broiler houses may vary depending upon the stage of the birds’ growth since the biomass of faeces and feather dandruff increase sharply during the fattening period. Moreover, when the fattened birds are collected for transportation to the slaughterhouse, the activity of workers who catch birds and put them into boxes transiently generates a lot of supplementary bioaerosol to which the forklift-truck operators, who load the boxes of chickens into the transportation, also may be exposed. Gathering temporal information about the quantity and the composition of bioaerosols is necessary to better understand the relationship between these factors and the adverse health symptoms of both workers and animals. Culture-dependent methods are by far the most widely used procedures for assessing the bacterial content of bioaerosols. The readout from this simple technique provides a quantitative measure of culturable bacteria and a low-resolution assessment of bacterial diversity. However, it is now widely accepted that our knowledge of microbial diversity is severely limited by the fact that the vast majority (> 90–99%) of naturally occurring microorganisms cannot be cultivated using standard techniques (Amann et al., 1995; DeLong and Pace, 2001). Moreover, dead airborne bacteria still have allergenic properties and are therefore also relevant to the occupational health assessment of total bacterial load. Epifluorescence, a method independent of culture, can be used to determine total bacterial concentration in air samples, but the technique is both time consuming and labour intensive, and samples must be analysed rapidly after collection. Real-time quantitative polymerase chain reaction (Q-PCR)—a molecular technique now widely applied in medical research areas where reproducible and accurate bacterial quantification are required—offers an attractive alternative method for quantifying total bacterial load while at the same time permitting assessment of the species-specific profile. In specific environmental situations where bioaerosol levels vary significantly over time, the composition may be highly dynamic. In such cases, the amenability, precision and reliability of Q-PCR should prove particularly advantageous.
The aim of this study was to measure exposure of total airborne bacteria and specific Staphylococcus species in poultry houses by Q-PCR methods and culture-dependent methods. In order to evaluate the influence of the age of birds on bioaerosol production, we have taken air samples at three different periods during the fattening process, in 12 poultry houses where the birds were confined but allowed to move freely on the litter. We also analysed air samples collected with personal devices that were placed both on workers who catch the birds and on drivers who collect the bird containers used to transport poultry to the slaughterhouse. The levels of airborne endotoxin, a component of Gram-negative bacteria, which is responsible for known acute and chronic respiratory effects, and total inhalable organic dust were also measured.
MATERIALS AND METHODS
Sites of sampling
Twelve poultry houses were each sampled four times during the fattening period. Individual poultry house size varied between 300 and 900 m2 (10 units of 300 m2, 1 of 600 m2 and 1 of 900 m2). Their floors were freshly covered with wood shavings at the beginning of bird fattening. Bird densities in 8 units were at 12.8 birds m−2 and in 4 units were at 17.3 birds m−2. All poultry houses had internal temperatures between 31–33°C during the first sampling session, 25–26°C during the second and 22–25°C during the third and fourth. The buildings were equipped with automatic feeding, watering and heating systems. All buildings have windows and have natural light cycle. Air samples were collected on the first or second day following the arrival of day-old chicks, at 24–27 days old, 1 day before the departure to slaughterhouse (40–53 days old) and during the handling of birds in preparation for transport to the slaughterhouse. In general, during the fattening period, a breeder is quite likely to spend several discrete periods of work inside the poultry house each day (between 1 and 3 h). The workers involved in the last stage catch the fattened chickens and load them into cages mounted in a rack standing on the floor. A catching crew consists of six catchers and a forklift-tractor operator who moves the racks of loaded cages from the poultry house to a trailer truck outside. For the three first sampling sessions, air was continuously sampled for 1–4 h during morning hours, at stationary points in the middle of the building without disturbing the birds. For the last sampling session (departure to slaughterhouse), an average of six workers per catching crew were equipped with a personal air sampler (placed in the proximity of the individual's breathing zone). At each stationary sampling spot, samples were collected in parallel onto three separate filter devices, by means of pocket pumps (MSA Escort Elf, Mine Safety Appliance Company, Pittsburgh, PA, USA or SKC pocket pump 210-1002, SKC Inc., Eighty Four, USA), set at a flow rate of 2.0 l min−1. Among the personal air samplers used during the chicken-catching process, usually two carried filters appropriate for endotoxin analysis, one for dust measurement and two for bacterial assessment. All workers participating to the study gave their consent. Five catchers and four drivers did not agree to participate and we have also lost four cassettes during the bird-catching process. Personal air samplers were allowed to run for 1 h. Airflow was calibrated before and after field sampling with a piston calibrator (DryCal DC-Lite, Bios International, Pompton Plains, NJ, USA). All stationary samples were taken 1.5 m above the floor.
Endotoxin sampling and analyse
Total airborne dust for endotoxin assessment was collected onto polycarbonate filters (37 mm diameter, 0.4 μm pore size), placed into a ready to use polystyrene cassette (endofree cassette, Aerotech Laboratories, Inc., Phoenix, AZ, USA). After sampling, cassettes were transported in a cold box to the laboratory within 3 h and were then stored at −20°C for 1–3 months to await endotoxin measurement. Endotoxins were extracted by shaking the filters at room temperature for 1 h in 10 ml of pyrogen-free water in a 50-ml conical polypropylene tube. The filter extraction solutions were vortexed vigorously prior to drawing a sample which was analysed for endotoxin content using a quantitative kinetic chromogenic limulus amoebocyte lysat assay (Biowhittaker, Cambrex Bio Science, Verviers, Belgium) at 37°C with an automated microtiter plate reader. Escherichia coli O55:B5 endotoxin (Biowhittaker) was used as a calibration standard to calculate endotoxin concentration in the experimental samples. Results were expressed in units of endotoxin per cubic metre of air.
Dust sampling
Inhalable dust was sampled on glassfiber filters and IOM heads (SKC Inc.). One field blank was taken on each day of measurement. Prior to and after use, the filters (taken out of the cassette holders) were weighed on an analytical balance (Mettler, 0.001 mg sensitivity).
Bacterial sampling on nutrient agar
Airborne bacteria were collected with an impactor (MAS-100 Eco, MBV, Vevey, Switzerland) at a flow rate of 100 l min−1. Air volumes of 10 l were sampled for non-specific bacteria; 20 l for Staphylococcus species. Total culturable bacteria were impacted onto Tryptone soya agar plates and Staphylococcus species onto Chapmann plates (all from Oxoïd, Basel, Switzerland). Plates were incubated at 30°C. All plates were checked daily for colony counts during 5 days. Results are expressed in colony forming units (CFU) per cubic metre of air. The most frequent Staphylococcus bacterial taxa were isolated and subsequently identified by using enzymatic test kits (API Staph, Biomérieux, France). All the colonies isolated (15) were identified as Staphylococcus xylosus by the API STA system; however, further molecular identification (Giammarinaro et al., 2005) carried out by Sabine Leroy (INRA—Centre de Clermont Theix, UR 454 Microbiologie, Qualité et Sécurité des Aliments, 63122 Saint-Genès-Champanelle, France) revealed that some strains were in fact Staphylococcus equorum. Gram-negative bacteria were not collected on selective nutrient agar since a pilot study has shown negative results.
Bacterial sampling for DNA analysis
Total airborne dust for bacterial DNA analysis was collected onto mixed cellulose ester filters (0.8-μm pore size 37 mm diameter, Millipore) contained in a 37-mm polystyrene cassettes (Millipore Corporation). These were stored at −20°C until analysis until processing could be achieved.
Filter processing and nucleic acid extraction
Genomic bacterial DNA used for standard concentration curves was extracted from pure cultures S. xylosus (a strain identified in our laboratory), using the FastDNA® SPIN Kit for Soil (Bio101, Carlsbad, CA, USA) and the protocol furnished with the kit. The amount of each extracted standard DNA was measured with a NanoDrop® ND-1000 spectrophotometer (NanoDrop Technologies). For the experimental samples, DNA was extracted directly from the filter using the FastDNA® SPIN Kit for Soil (Bio101), after mechanical lysis of bacterial wall material. The extraction was done in accordance with the manufacturer instructions, substituting the filter for the recommended 500 μg soil sample. The DNA was eluted in 200 μl Qiagen EB buffer.
Real-time polymerase chain reaction amplification and quantification
All polymerase chain reaction (PCR) assays were performed on a RotorGene 3000 (Corbett Research, Mortlake, Australia). The calibration curve was generated using the RotorGene software, version 6.0.16 and was used in conjunction with the software to calculate the initial target bacteria number in the sample.
Standard curve.
Serial dilutions of S. xylosus genomic DNA were used as standards for determining bacterial and Staphylococcus number by real-time PCR. The number of equivalent S. xylosus of the Q-PCR standards was calculated with the following equation: genome weight in Dalton, W = [%GC × total length] × 618.4/100 + [(100 − %GC) × total length] × 617.4/100 + 36. Where total length = 2.9 × 103 kb (Dordet-Frisoni et al., 2007) and as S. xylosus is a low GC content bacteria, the %GC was arbitrarily fixed at 35%. Genome copies per nanogram of DNA = (NL/W) × 10−9, where NL is the Avogadro constant (6.02 × 1023 molecules per mol). The calibration curve was generated with the RotorGene software, version 6.0.16. Using the calibration curve, the initial number of equivalent S. xylosus in the PCR was calculated by the RotorGene software. Both the total bacterial load and the staphylococcal load were expressed in equivalents S. xylosus.
Total bacterial load assessment.
A 466-bp fragment of the bacterial 16S rDNA 331–797 (according to E. coli position) was amplified with a universal primers and probe set (Nadkarni et al., 2002). The PCR was performed in a total volume of 20 μl using the qPCR Core kit No ROX (Eurogentec) with 2.5 mM MgCl2, 0.4 mM dNTP mix, 0.1 μM forward primer (5′-TCCTACGGGAGGCAGCAGT-3′), 0.1 μM reverse primer (5′-GGACTACCAGGGTATCTAATCCTGTT-3′), 0.1 μM probe (5′-HEX-CGTATTACCGCGGCTGCTGGCAC-BHQ1-3′) and 0.5 U per reaction of Hot Gold Star polymerase. Two microlitres of the sample was added to the PCR mix. The amplification was carried out under the following conditions: 95°C for 10 min and 50 cycles of 95°C for 15 s and 60°C for 1 min, with acquisition of the fluorescence on the JOE canal at the end of each 60°C elongation step. No-template controls with Qiagen EB buffer instead of template DNA were included in each run. As Staphylococcus species was the most frequent cultivable bacteria found in poultry houses, serial dilutions of S. xylosus DNA were used as the standard for determining bacterial number by real-time PCR. The standards and the samples were included in duplicate in each run.
Staphylococcus species load assessment.
A 370-bp fragment of the Staphylococcus species tuf gene, which encodes the elongation factor Tu, was amplified with a primer set specific for the Staphylococcus genus (Martineau et al., 2001). The PCR was performed in a total volume of 20 μl using the qPCR MasterMix Plus for SYBR® Green I No ROX (Eurogentec) with 10 μl of Master Mix, 0.2 μM of forward primer TStaG422 (5′-GGCCGTGTTGAACGTGGTCAAATCA-3′) and 0.2 μM of reverse primer TstaG765 (5′-TIACCATTTCAGTACCTTCTGGTAA-3′, I = inosine). Two microlitres of DNA was added to the PCR mix. The amplification was carried out under the following conditions: 50°C for 2 min, 95°C for 10 min and 50 cycles of 95°C for 15 s and 55°C for 20 s and 72°C for 40 s, with acquisition of the fluorescence on the SYBR channel at the end of each elongation step. No-template controls with Qiagen EB buffer instead of template DNA were included in each run. Serial dilutions of S. xylosus DNA were used as the standard for determining bacterial number by real-time PCR. Duplicate aliquots of the standards and the samples were included in each PCR run.
Statistical methods
The effect of stages of fattening and density of birds on bioaerosols (log-transformed data) was tested with an analysis of variance. Assumptions underlying the models were checked beforehand (normality tested with Shapiro test and homogeneity of variance tested with Bartlett test). Correlation between the different bioaerosols was tested with Pearson correlation tests. All statistics were done by using Systat software (SYSTAT Software Inc. products, Richemond, Canada) and R free software. The data are presented by geometric mean values ± geometric standard deviation (GSD).
RESULTS
The geometric mean airborne concentration of cultivable Staphylococcus species circulating in the air of poultry houses containing 1- to 2-day-old chickens was 19 (±1.2) × 103 CFU m−3, with this number representing ∼90% of the total cultivable bacteria present on the nutrient plates (data not shown). Importantly, it was found to be impossible to apply standard culture-dependent methods using impaction of bacteria onto nutrient media after the first stage of the fattening period since all subsequent samples after this stage were so oversaturated with bacteria that colonies could not be counted, even when the sampling duration was very short (5 s corresponding to <5 l of air).
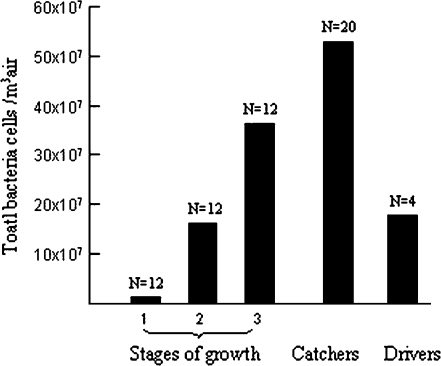
The estimated Staphylococcal species load, as quantified by Q-PCR during the first stage of sampling [GM ± GSD = 88 (±5.9) × 103 m−3] (Fig. 1), is ∼4-fold higher than the value obtained by culture of impacted bacteria. Furthermore, this estimate represents a mean value of only 0.75% (±1%) (Table 1) of the total bacterial load as measured by Q-PCR (N = 59) (Fig. 2). The comparative analysis by Q-PCR of Staphylococcal versus total bacterial load suggests that this ratio changes during the fattening period (Table 1). Both the number of total bacterial cells and Staphylococcus species cells measured by Q-PCR increase massively during the fattening period, as do the inhalable dust and endotoxin levels (Figs 1– 4). Analysis of variance show that the stage of fattening has a significant influence on the quantity of all bioaerosols, while the bird density in the poultry house has no effect (Table 2). We found significant positive correlations between inhalable dust level and the parameters of endotoxin load, number of total bacterial cells estimated by Q-PCR, number of Staphylococcus cells estimated by Q-PCR (respectively, Pearson correlation = 0.573, 0.451 and 0.603; P < 0.02, N = 42). During the load to the slaughterhouse, the workers who directly handle the birds are exposed to higher levels of bioaerosol than are the forklift drivers (Figs 1– 4). We found that the geometric mean inhalable dust concentration for bird catchers was of 26.3 ± 1.9 mg m−3. Eighty-five per cent (11 of 13) of the catchers were exposed to dust level values >10 mg m−3. The mean exposure level of catchers to endotoxin concentration is 6198 ± 2.3 EU m−3 air. This value is 6-fold more elevated than the Swiss occupational recommended value (1000 EU m−3). As measured by Q-PCR, the geometric mean exposure levels of bird catchers to total bacteria and Staphylococcus species are, respectively, 53 (±2.6) × 107 cells m−3 and 62 (±1.9) × 106 cells m−3.
Fig. 1.
Geometric mean of Staphylococcus species (estimated by Q-PCR) per cubic metre of air sampled at different stages of growth (Stage 1, 2 and 3; see details in text) and within the breathing zone of bird's catcher and driver as they caught the birds ready to be transported for slaughter, in 12 poultry houses. Numbers beside the bars indicate the sample size.
Table 1.
Airborne staphylococci as percentage of total bacteria, estimated by Q-PCR, in 12 poultry houses, at different stages of fattening period
| Stage of fattening | 1 (1–2 days old) | 2 (24–27 days old) | 3 (40–53 days old) |
| Staphylococci as percentage of total bacteria | 0.75 ± 1.2 | 9.6 ± 2.4 | 11 ± 1.5 |
Fig. 2.
Geometric mean of total bacteria (estimated by Q-PCR) per cubic metre of air sampled at different stages of growth (Stage 1, 2 and 3; see details in text) and within the breathing zone of bird's catcher and driver as they caught the birds ready to be transported for slaughter, in 12 poultry houses. Numbers beside the bars indicate the sample size.
Fig. 4.
Geometric mean of dust concentration per cubic metre of air sampled at different stages of growth (Stage 1, 2 and 3; see details in text) and within the breathing zone of bird's catcher and driver as they caught the birds ready to be transported for slaughter, in 12 poultry houses. Numbers beside the bars indicate the sample size.
Table 2.
Analysis of variance: effects of stages of fattening and density of birds on the values of bioaerosols concentrations, estimated by Q-PCR method, in 12 Swiss poultry houses
| Source of variation | d.f. | Mean sum of square | F-ratio | P |
| Response: airborne endotoxin, R2 = 0.592 | ||||
| Stages | 2 | 8.882 | 20.164 | <0.001 |
| Density | 1 | 0.018 | 0.041 | 0.840 |
| Stage × density | 2 | 0.043 | 0.097 | 0.907 |
| Residuals | 30 | 0.44 | ||
| Response: airborne Staphylococcus species, R2 = 0.676 | ||||
| Stages | 2 | 123.332 | 23.106 | <0.001 |
| Density | 1 | 18.614 | 3.487 | 0.072 |
| Stage × density | 2 | 0.869 | 0.163 | 0.851 |
| Residuals | 29 | 5.338 | ||
| Response: total airborne bacteria species, R2 = 0.830 | ||||
| Stages | 2 | 33.208 | 62.734 | <0.001 |
| Density | 1 | 0.146 | 0.276 | 0.603 |
| Stage × density | 2 | 0.524 | 0.989 | 0.384 |
| Residuals | 30 | 0.529 | ||
| Response: inhalable dust, R2 = 0.792 | ||||
| Stages | 2 | 20.416 | 39.972 | <0.001 |
| Density | 1 | 0.007 | 0.013 | 0.910 |
| Stage × density | 2 | 0.133 | 0.261 | 0.773 |
| Residuals | 24 | 0.511 |
Fig. 3.
Geometric mean of endotoxin concentration per cubic metre of air sampled at different stages of growth (Stage 1, 2 and 3; see details in text) and within the breathing zone of bird's catcher and driver as they caught the birds ready to be transported for slaughter, in 12 poultry houses. Numbers beside the bars indicate the sample size.
DISCUSSION
The results of the present study showed that as expected, levels of airborne bacteria and endotoxin as well as inhalable dust increase drastically throughout the growth of the enclosed chickens. This is not surprising since total biomass (number of birds × weight) and consequently skin debris, broken feather barbules, aerosolized feed and poultry excreta also increase drastically during this period. However, significant changes in parameters such as temperature inside the poultry houses during the different stages of fattening may alter the composition of the bioaerosol as well as changing the overall rate of bacterial growth. The increase of activity during the handling and loading of birds for departure to slaughterhouse explain the increase of bioaerosol level compared to the level measured the day before.
Results from Q-PCR methods are consistent with other estimates of poultry house bacterial load, obtained using epifluorescence methods (Radon et al., 2001). High levels of bioaerosol could significantly affect pulmonary functions of poultry workers (Olenchock et al., 1982; Clark et al., 1983; Jones et al., 1984). Indeed, several epidemiological studies suggest that workers in poultry houses do experience significantly higher rates of acute symptoms of pulmonary stress than workers engaged in a broad cross-section of other employment (Morris et al., 1991; Schwartz et al., 1992; Radon et al., 2001; Rylander and Carvalheiro, 2006). One attempt to ascertain the dose at which poultry house dust exposure is associated with significant impairment of pulmonary function estimated this at 2.4 mg m−3 for total airborne dust and 614 EU m−3 for endotoxin (Donham et al., 1990, 2000). Our data suggest that essentially all the forklift drivers as well as the catchers who participated in our study widely exceeded the exposure–response threshold recommended for dust and endotoxin levels in these two studies. To date, most of the recommendation limit values for total bacteria are given in terms of CFU m−3 air. However, one of the most important points that emerge from our comparative study of different methods to assess temporal changes in bacterial load during the poultry production process is that the traditional culture-dependent methods are totally inappropriate to the task due to the high levels of bacteria produced in such environments. Since the relationship between the number of cultivable bacteria and the total number of bacteria is often unknown, it is important to acquire data that allow one to determine what the equivalent threshold limit value as estimated by Q-PCR should be. The comparative values we obtained for quantification of staphylococci species in the enclosures holding young chicks suggest that in general, Q-PCR is likely to give estimates of bacterial numbers at least 3- to 4-fold higher than obtained by traditional culture methods. Thus, the suggested value for a reasonable threshold limit expressed in units measured by Q-PCR should take account of this correction.
The mean quantities obtained by our Q-PCR analyses (5.3 × 108 cells m−3) were in the same range as those obtained in a study of poultry houses in Denmark (Nielsen and Breum, 1995), made under similar conditions (catchers equipped with personal samplers), but using epifluorescence methods (7.0 × 108 cells m−3). The Danish study reported that microscopy showed that spherical bacteria, of mainly Staphylococcus species, dominated the bacterial community. A predominance of airborne Gram-positive bacteria (especially coagulase-negative staphylococci) was also demonstrated in another study that reported bacterial concentration in the ambient air of poultry slaughterhouses (Haas et al., 2005). The discrepancy between the ratio of staphylococci to other species as estimated by air impaction onto nutrient media compared to the Q-PCR method is likely due to a combination of effects, with Q-PCR detecting other bacterial strains that are either dead or viable but uncultivable. It is also possible that the Staphylococcus species detected by culture are able to outcompete other more fastidious bacteria and prevent their growth.
These potential explanations underline the limitations of culture-dependent methods to assess the airborne bacterial load, while several other considerations suggest that further studies are needed to assess the risks to human health of such high concentrations of Staphylococcus species in the ambient air. In particular, peptidoglycan, the major component of Gram-positive cell walls, shares some pro-inflammatory properties with endotoxins and may induce respiratory symptoms (Johannsen et al., 1990; Mattsson et al., 1993; Zhiping et al., 1996; Dziarski et al., 2001; Douwes et al., 2003; Sigsgaard et al., 2005; Visser et al., 2005). It has even been proposed that peptidoglycan should be regarded as an endotoxin in its own right (Myhre et al., 2006).
CONCLUSION
To conclude, poultry house workers, and most particularly those employed in the task of bird catching, are regularly exposed to very high levels of airborne bacteria, endotoxin and dust. The poultry industry should be made aware of the potential respiratory health effect of occupational exposure to bioaerosol and preventive measures to protect workers should be taken despite the present lack of well-defined legal limit levels concerning this biological hazard.
We have demonstrated that the use of Q-PCR to assess total or species-specific airborne bacterial load seems to be a promising tool, which is less time- and labour-consuming than microscopic methods with the added advantage that the same samples can also be analysed by complementary molecular techniques to obtain information about their species diversity. The need to evaluate the validity of Q-PCR as a tool for quantitative bioaerosol exposure assessment is now crucial.
FUNDING
Swiss National Foundation (3200BO-104246) to A.O.
Acknowledgments
Thanks to Moira Cockell for corrections and helpful comments on the draft. We greatly appreciate the support of owners and staff of the poultry houses that cooperated in this study.
References
- Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in-situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–69. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S, Rylander R, Larsson L. Airborne bacteria, endotoxin and fungi in dust in poultry and swine confinement buildings. Am Ind Hyg Assoc J. 1983;44:537–41. doi: 10.1080/15298668391405265. [DOI] [PubMed] [Google Scholar]
- DeLong EE, Pace NR. Environmental diversity of bacteria and archaea. Syst Biol. 2001;50:470–8. [PubMed] [Google Scholar]
- Donham KJ, Leistikow B, Merchant J, et al. Assessment of United-States poultry worker respiratory risks. Am J Ind Med. 1990;17:73–4. doi: 10.1002/ajim.4700170118. [DOI] [PubMed] [Google Scholar]
- Donham KJ, Cumro D, Reynolds SJ, et al. Dose-response relationships between occupational aerosol exposures and cross-shift declines of lung function in poultry workers: recommendations for exposure limits. J Occup Environ Med. 2000;42:260–9. doi: 10.1097/00043764-200003000-00006. [DOI] [PubMed] [Google Scholar]
- Dordet-Frisoni E, Talon R, Leroy S. Physical and genetic map of the Staphylococcus xylosus C2a chromosome. FEMS Microbiol Lett. 2007;266:184–93. doi: 10.1111/j.1574-6968.2006.00538.x. [DOI] [PubMed] [Google Scholar]
- Douwes J, Thorne P, Pearce N, et al. Bioaerosol health effects and exposure assessment: progress and prospects. Ann Occup Hyg. 2003;47:187–200. doi: 10.1093/annhyg/meg032. [DOI] [PubMed] [Google Scholar]
- Dziarski R, Wang QL, Miyake K, et al. MD-2 enables toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J Immunol. 2001;166:1938–44. doi: 10.4049/jimmunol.166.3.1938. [DOI] [PubMed] [Google Scholar]
- Giammarinaro P, Leroy S, Chacornac JP, et al. Development of a new oligonucleotide array to identify staphylococcal strains at species level. J Clin Microbiol. 2005;43:3673–80. doi: 10.1128/JCM.43.8.3673-3680.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D, Posch J, Schmidt S, et al. A case study of airborne culturable microorganisms in a poultry slaughterhouse in Styria, Austria. Aerobiologia. 2005;21:193–201. [Google Scholar]
- Johannsen L, Toth LA, Rosenthal RS, et al. Somnogenic, pyrogenic, and hematologic effects of bacterial peptidoglycan. Am J Physiol. 1990;258:182–6. doi: 10.1152/ajpregu.1990.258.1.R182. [DOI] [PubMed] [Google Scholar]
- Jones W, Morring K, Olenchock SA, et al. Environmental study of poultry confinement buildings. Am Ind Hyg Assoc J. 1984;45:760–6. [PubMed] [Google Scholar]
- Martineau F, Picard FJ, Ke D, et al. Development of a PCR assay for identification of staphylococci at genus and species levels. J Clin Microbiol. 2001;39:2541–7. doi: 10.1128/JCM.39.7.2541-2547.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson E, Verhage L, Rollof J, et al. Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumour necrosis factor-alpha, interleukin-1 beta and interleukin 6. FEMS Immunol Med Microbiol. 1993;7:281–8. doi: 10.1111/j.1574-695X.1993.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Morris PD, Lenhart SW, Service WS. Respiratory symptoms and pulmonary function in chicken catchers in poultry confinement units. Am J Ind Med. 1991;19:195–204. doi: 10.1002/ajim.4700190207. [DOI] [PubMed] [Google Scholar]
- Myhre AE, Aasen AO, Thiemermann C, et al. Peptidoglycan—an endotoxin in its own right? Shock. 2006;25:227–35. doi: 10.1097/01.shk.0000191378.55274.37. [DOI] [PubMed] [Google Scholar]
- Nadkarni MA, Martin FE, Jacques NA, et al. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148:257–66. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- Nielsen BH, Breum NO. Exposure to air contaminants in chicken catching. Am Ind Hyg Assoc J. 1995;56:804–8. doi: 10.1080/15428119591016638. [DOI] [PubMed] [Google Scholar]
- Olenchock SA, Lenhart SW, Mull JC. Occupational exposure to airborne endotoxins during poultry-processing. J Toxicol Environ Health. 1982;9:339–49. doi: 10.1080/15287398209530166. [DOI] [PubMed] [Google Scholar]
- Radon K, Danuser B, Iversen M, et al. Respiratory symptoms in European animal farmers. Eur Respir J. 2001;17:747–54. doi: 10.1183/09031936.01.17407470. [DOI] [PubMed] [Google Scholar]
- Rylander R, Carvalheiro MF. Airways inflammation among workers in poultry houses. Int Arch Occup Environ Health. 2006;79:487–90. doi: 10.1007/s00420-005-0072-5. [DOI] [PubMed] [Google Scholar]
- Sauter EA, Petersen CF, Parkinson JF, et al. Microbial buildup in air and dust during brooding and rearing of chickens. Poult Sci. 1981;60:1724–1725. [Google Scholar]
- Schwartz DA, Landas SK, Lassise DL, et al. Airway injury in swine confinement workers. Ann Intern Med. 1992;116:630–635. doi: 10.7326/0003-4819-116-8-630. [DOI] [PubMed] [Google Scholar]
- Sigsgaard T, Bonefeld-Jorgensen EC, Hoffmann HJ, et al. Microbial cell wall agents as an occupational hazard. Toxicol Appl Pharmacol. 2005;207:310–9. doi: 10.1016/j.taap.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Thelin A, Tegler O, Rylander R. Lung reactions during poultry handling related to dust and bacterial-endotoxin levels. Eur J Respir Dis. 1984;65:266–71. [PubMed] [Google Scholar]
- Visser L, De Heer HJ, Boven L, et al. Proinflammatory bacterial peptidoglycan as a cofactor for the development of central nervous system autoimmune disease. J Immunol. 2005;174:808–16. doi: 10.4049/jimmunol.174.2.808. [DOI] [PubMed] [Google Scholar]
- Zhiping W, Malmberg P, Larsson BM, et al. Exposure to bacteria in swine-house dust and acute inflammatory reactions in humans. Am J Respir Crit Care Med. 1996;154:1261–6. doi: 10.1164/ajrccm.154.5.8912733. [DOI] [PubMed] [Google Scholar]