Abstract
Franz Sikora found the first specimen and type of the recently extinct Hadropithecus stenognathus in Madagascar in 1899 and sent it to Ludwig Lorenz von Liburnau of the Austrian Imperial Academy of Sciences. Later, he sent several more specimens including a subadult skull that was described by Lorenz von Liburnau in 1902. In 2003, some of us excavated at the locality and found more specimens belonging to this species, including much of a subadult skeleton. Two frontal fragments were found, and these, together with most of the postcranial bones, belong to the skull. CT scans of the skull and other jaw fragments were made in Vienna and those of the frontal fragments at Penn State University. The two fragments have been reunited with the skull in silico, and broken parts from one side of the skull have been replaced virtually by mirror-imaged complete parts from the other side. The parts of the jaw of another individual of a slightly younger dental age have also been reconstructed virtually from CT scans with mirror imaging and by using the maxillary teeth and temporomandibular joints as a guide to finish the reconstruction. Apart from forming a virtual skull for biomechanical and systematic analysis, we were also able to make a virtual endocast. Missing anterior pieces were reconstructed by using part of an endocast of the related Archaeolemur majori. The volume is 115 ml. Hadropithecus and Archaeolemur seem to have had relatively large brains compared with the other large-bodied subfossil lemurs.
Keywords: computed tomography, Madagascar
The Austrian professional fossil collector (and trader), Franz Sikora, collected fossils from Andrahomana cave in southeastern Madagascar at the end of the 19th Century. For a review of the site and the collections from it, see Burney et al. (1). Sikora sent some specimens to paleontologist Ludwig Lorenz von Liburnau at the Imperial Austrian Academy of Sciences in Vienna (Kaiserliche Akademie der Wissenschaften, Vienna), and among them was a mandible that Lorenz thought belonged to an anthropoid primate. He named it Hadropithecus stenognathus (2). In a subsequent publication, he figured a cranium found by Sikora and included sketches of superior and inferior views of it (3). Based on the photographs and without seeing the specimen itself, he named it Pithecodon sikorae. This skull was, in fact, a younger individual of the same species to which the jaw belonged, as Lorenz von Liburnau quickly realized when he received the specimen from Sikora. He published on it and several other upper and lower jaw fragments and limb bones in 1902 (4). Charles Lamberton of the Académie Malgache in Antananarivo collected the only other known Hadropithecus skull from Tsirave in southwest Madagascar in 1931. He described it and at the same time refigured Lorenz von Liburnau's (4) Plate 1 that illustrated the first cranial remains (5). The latter skull is from an older individual with extremely worn teeth. Tattersall (6) gives a full account of all this material together with much cranial material of the better known Archaeolemur species. Sikora's fossils from Madagascar were transferred in 1900 from the Imperial Austrian Academy of Sciences first to the zoological department of the Natural History Museum of Vienna and later, in 1934, to the geological-paleontological department.
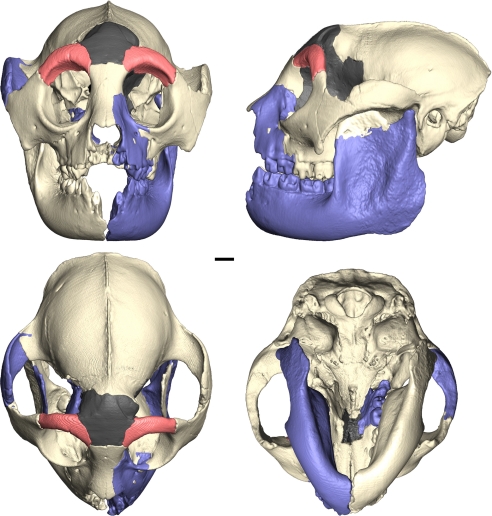
Much of a subadult skeleton belonging to this species was found during new excavations at Andrahomana in 2003 (7). Two frontal fragments were found, and these, together with most of the postcranial bones, belong to the skull. The right side only of the type mandible (NHMW1934 IV 1/1) was figured by von Liburnau, and it has since been lost. Only the left side with three molars (NHMW1934 IV 1/2) remains of the type. Hadropithecus is one of the most poorly known extinct Madagascan lemurs, but with the new fossils rejoined with the originals after more than a century, we have an opportunity to advance our knowledge of it. We decided to reconstruct the Vienna cranium (NHMW 1934 IV 1) as accurately as we could using medical CT scans together with scans of a partial right mandible (NHMW1934 IV 2/1a) and its left counterpart (NHMW1934 IV 2/1b).
Results
The finished reconstruction does not include missing parts of the anterior dentition and the premaxilla. In the future, these could be reconstructed from the Tsirave skull. An isolated upper canine found in 2003 that probably belonged to the Vienna skull was destroyed for ancient DNA analysis, but no DNA was found. It was inadvertently destroyed before a security cast could be made. A second upper molar was destroyed for enamel structure analysis (8), and a safety mold of this was scanned for use in the reconstruction to replace its damaged antimere.
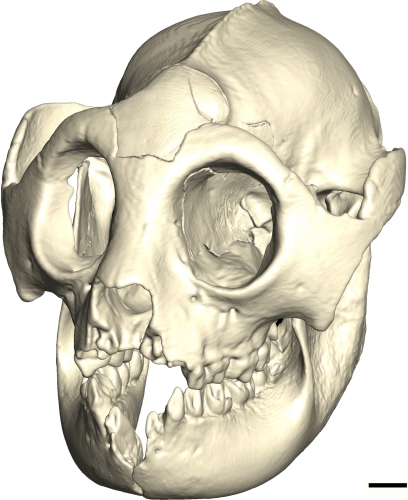
The reconstruction shows a very short face hafted onto a globular braincase (Figs. 1 and 2). In superior view, with its temporal crest, and curved zygomatic arches with strong postorbital constriction framing a large temporal fossa, the skull has a resemblance to Australopithecus (or Paranthropus) boisei skulls. This resemblance does not show in the details of the dentition however, because A. boisei has flattish, thick-enameled cheek teeth that increase in area distally, whereas H. stenognathus has enlarged middle parts of the tooth row with cuspidate teeth that wear quickly to form complex, unguliform enamel ridges. Furthermore, Hadropithecus lacks the thick enamel with heavy decussation throughout that is characteristic of Australopithecus boisei (and, incidentally, Archaeolemur) (8, 9). Clearly both Australopithecus and Hadropithecus needed large masticatory muscles with a reduced emphasis on the anterior dentition, but they must have eaten foods with substantially different material properties.
Fig. 1.
Frontal, superior, inferior, and left lateral views of the reconstruction of the skull of Hadropithecus. The white portions are original fossils described by Lorenz von Liburnau, red colored areas are frontal fragments found in 2003, blue regions are mirror imaged from opposite side of this skull, and the gray section was reconstructed with wax from a 3D stereolithography print. (Scale bar: 10 mm.)
Fig. 2.
Three-quarters view of skull reconstruction with all reconstructed sections uniformly colored. (Scale bar: 10 mm.)
The skull shows maxillary, sphenoid, and extensive frontal paranasal sinuses. These latter, like those of some New World monkeys (10, 11), have developed convergently with those of some anthropoid apes.
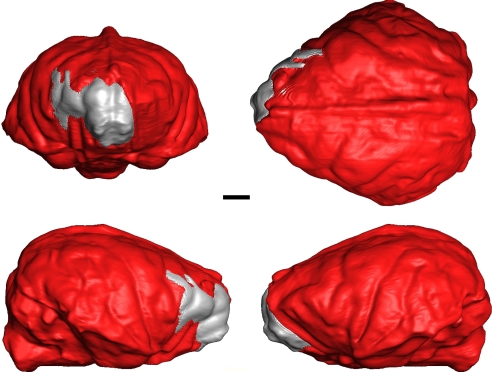
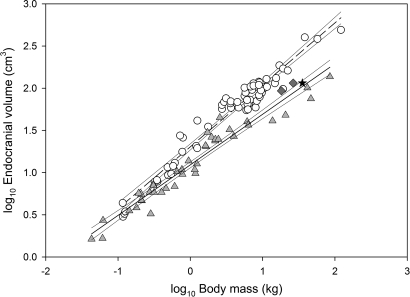
The endocranium is relatively large compared with other strepsirhines. The volume of the endocranial cavity, with the most frontal part based on Archaeolemur edwardsi is 115.0 ml (Fig. 3). A displacement volume of the Tsirave skull made by W.L.J. is 106 ml. Conventional least-squares regression analysis shows that when the recently extinct lemurs are included in the sample, the two archaeolemurids have just above average cranial capacities relative to body mass for strepsirhines, and they are small for anthropoids (but see ref. 12). Compared to the other large-bodied subfossil strepsirhines, Archaeolemur appears to be significantly encephalized. Daubentonia madagascariensis and Archaeolemur species are the only extant or extinct strepsirhines with a cranial capacity that is as large as a haplorhine of the same body mass (Fig. 4).
Fig. 3.
Frontal, lateral, and superior views of the endocranial reconstruction of Hadropithecus with gray portion from Archaeolemur (AMNH 30007). (Scale bar: 10 mm.)
Fig. 4.
Log–log plot of endocranial volume against body mass for extant primates. Least-squares linear regressions were calculated for haplorhines, open circles (log10ECV = 0.737 × log10BM + 1.305; r2 = 0.953) and strepsirhines, triangles (log10ECV = 0.594 × log10BM + 1.090; r2 = 0.948). Hadropithecus is indicated by the black star; Archaeolemur species are represented by gray diamonds; thin lines represent 95% confidence intervals. In view of these extremely high correlation coefficients, model II regressions would differ very little from the least-squares lines.
This reconstruction, together with correctly associated limb bones (7, 13) means that we have a reasonable idea of the proportions (skull, brain, teeth, thorax, and limbs) of a single individual of this peculiar strepsirhine primate. Full or partial skeletons of single individuals can, in some respects, be more important than the same number of parts from several individuals, because comparisons with whole skeletons of living taxa can be made more easily.
The locomotor adaptations of this species have been the subject of much speculation, but reconstruction of the true locomotion has been hampered by misattributions of limb bones to Hadropithecus and an overreliance on analogies to living cercopithecoids (14). This species was a mostly terrestrial quadruped that could undoubtedly climb but that shows no signs of suspensory or leaping adaptations (7, 13, 15). The size of the semicircular canals can be used to corroborate the locomotor reconstructions of extinct primates, and in the case of Hadropithecus, they show that it was less agile than living Old World monkeys (16).
One interesting comparison is with the closely related Archaeolemur. The species of this genus have very enlarged anterior teeth, rather than reduced ones as in Hadropithecus. They also have a unique shearing mechanism comprising three upper and lower premolars and have clearly bilophodont molars that resemble those of Old World Monkeys. These closely related genera appear to have very different feeding adaptations. The other comparison, suggested by Jolly (17) and generally endorsed by Tattersall (6), is with Theropithecus gelada. Jolly used limb proportions from Lamberton (5) that were wrongly attributed, and so that part of his argument can be discounted, but he pointed out that both species had relatively small, vertical incisors and enlarged thick-enameled cheek teeth that wear flat and make complex ridges of infolded enamel. T. gelada are mostly graminivorous, with grass blades, seeds, flowers, and rhizomes eaten, although they do eat other fall-back plants (18). Rafferty et al. (19) tentatively concluded, based on conventional microwear analysis of only two teeth, that Hadropithecus was decidedly not like the modern gelada baboon but probably did feed on hard objects. Godfrey et al. (20), using another microwear method with a sample of nine teeth, also concluded that it differed significantly from Theropithecus and that they were hard object feeders. J. R. Scott, et al. (unpublished work), using scale sensitive fractal analysis of five specimens of Hadropithecus tooth replicas, also supported a hard-object feeding regime. It is worth noting that δ13C values from five Hadropithecus specimens (from four different sites in southern and western Madagascar) give a clear indication of a reliance on C4/CAM plants (8, 9), so that grass could have been part of the diet of this species. Exceptionally high δ15N values (9) may suggest a preference for CAM over C4 plants, however. Codron et al. (21, 22) have shown that succulent plants (many of which are CAM) in South Africa can be δ15N-enriched, and Loudon et al. (23) report heavy δ15N as well as δ13C values for Lemur catta at Tsimanampesotse, where CAM plant consumption is much greater than at Beza Mahafaly. Further research on the stable isotopes of succulent CAM plants and grasses in southern Madagascar will undoubtedly help to clarify likely dominant components of the diet of Hadropithecus. The degree to which heavy microwear pitting reflects high exogenous grit as opposed to the consumption of hard foods per se will also require further exploration (9); the dental microstructure of Hadropithecus is better suited to resist tough foods than to resist hard foods (see ref. 24).
Methods
The original material in the Vienna Museum of Natural History was scanned using a Philips CT scanner in helical mode. Scan parameters for the cranium (NHMW 1934 IV 1) were: matrix of the dataset x/y/z = 512/512/221, voxel size = 0.29297/0.29297/0.4 mm, 140 kV, 53 mA, ear kernel. Scan parameters for the mandibular fragments (NHMW1934 IV 2/1a,b) were: matrix of the dataset x/y/z = 512/512/194, voxel size = 0.21094/0.21094/0.4 mm, 120 kV, 108 mA, inner ear kernel. The two orbital processes of the frontal bones were scanned on the HD350 medical CT scanner (Universal Systems) at the Center for Quantitative Imaging at Pennsylvania State University. They were then repositioned in silico without any scaling according to anatomical clues (25) into the Vienna cranium by using the imaging program Amira 3.1.1 (Visage Imaging). The following parts were reconstructed based on mirror imaging: right zygomatic arch; left facial portion with premolars; right second molar; right mandible, left coronoid process. The virtual reconstruction of the cranium was then produced as a real 3D model by using a Z-Corp 3-D printer (Z Corporation) in the Department of Engineering at Pennsylvania State University. The missing parts of the frontal bone over the frontal sinus, the ethmoid and sphenoid bones forming the medial wall of the orbit and temporal fossa, and small portions of the palate and zygomatic arch were conventionally reconstructed by using modeling wax and following the contours of the surrounding intact bones. The 3D model with wax reconstruction was CT scanned by using the HD350 CT at Pennsylvania State University, and the wax portion was extracted and repositioned into the Vienna cranium, again without scaling. The coronoid process of the right mandible that is figured in Lorenz von Liburnau's Plate 1 (NHMW1934 IV 2/1a) (4) has been missing for some time, perhaps even before the transfer from the Academy to the Museum or from the zoological to the geological-paleontological collection of the Museum, but it is still present on the much more fragmentary left side (NHMW1934 IV 2/1b). The coronoid from the left side was extracted, mirror imaged, and repositioned onto the right side mandible in silico in Amira 3.1.1 to create a composite right hemimandible. This entire reconstructed hemimandible was then mirror imaged to create a corresponding left-side mandible. Each of these mandibular pieces was articulated with the cranium by using the temporomandibular joints and upper teeth as a guide to proper occlusion. The missing anterior teeth and some apparent slight bilateral distortion of the cranium prevent perfect symmetry in the mandibular reconstruction. The endocranial surface of the cranium was selected using Amira 3.1.1 to produce a virtual endocast. The very anterior part of the braincase is missing, and so we used laser scan data of the anterior endocast of Archaeolemur edwardsi (AMNH 30007), which is of nearly identical size and shape to that of the Vienna skull. The volume of the endocast was calculated from the 3D reconstruction in Amira 3.1.1 by using the SurfaceArea measuring tool. Conventional least-squares regression analysis was performed by using body mass and endocranial volume data for a sample of modern primates from Kirk (26).
Acknowledgments.
We thank Avrami Grader and Phil Halleck of the Pennsylvania State Center for Quantitative Imaging and Dr. Herwig Imhof and Sonja Plischke from the University Clinic for Radiology, Medical University of Vienna, for help with the CT scanning. Wade Shumaker and Bruce Knoll helped with 3D printing at various stages of the reconstruction. Harry Jerrison kindly provided the scan data for the Archaeolemur endocast. Ian Tattersall and Bill Sanders provided constructive comments that improved this manuscript. This research was supported by National Science Foundation Grants BCS-0129185, BCS-0237338, SBR-9617286, and BNS-8823083, The Leakey Foundation, Pennsylvania State and Portland State Universities, and European Union Grant MRTN-CT-2005-019564.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 10639.
References
- 1.Burney DA, et al. New findings at Andrahomana Cave, Southeastern Madagascar. J Cave Karst Stud. 2008 in press. [Google Scholar]
- 2.Lorenz von Liburnau LR. A fossil anthropoid from Madagascar (translated from German) Anz Kais Akad Wiss Wien. 1899;36:255–257. [Google Scholar]
- 3.Lorenz von Liburnau LR. More extinct primates from Madagascar (translated from German) Denkschr kais Akad Wiss Wien. 1901;70:1–15. [Google Scholar]
- 4.Lorenz von Liburnau LR. On Hadropithecus stenognathus (translated from German) Denkschr kais Akad Wiss Wien. 1902;72:243–254. [Google Scholar]
- 5.Lamberton C. Contribution to the knowledge of the subfossil fauna of Madagascar. Note III. Hadropithecus (translated from French) Bull Acad Malgache. 1938;27:75–139. [Google Scholar]
- 6.Tattersall I. Cranial aanatomy of the Archaeolemurinae (Lemuroidea, Primates) Anthropol Papers Am Mus Nat Hist. 1973;52:1–110. [Google Scholar]
- 7.Godfrey LR, et al. New discoveries of skeletal elements of Hadropithecus stenognathus from Andrahomana Cave, southeastern Madagascar. J Hum Evol. 2006;51:395–410. doi: 10.1016/j.jhevol.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Godfrey LR, et al. New insights into old lemurs: The trophic adaptations of the Archaeolemuridae. Int J Primatol. 2005;26:825–854. [Google Scholar]
- 9.Godfrey LR, Crowley BE, Muldoon KM, King SJ, Burney DA. The Hadropithecus conundrum. Am J Phys Anthropol Suppl. 2008;46:105. [Google Scholar]
- 10.Hershkovitz P. Living New World Monkeys (Platyrrhini) with an Introduction to Primates. Chicago: University of Chicago Press; 1977. [Google Scholar]
- 11.Rossie JB. Ontogeny and homology of the paranasal sinuses in Platyrrhini (Mammalia: Primates) J Morphol. 2006;267:1–40. doi: 10.1002/jmor.10263. [DOI] [PubMed] [Google Scholar]
- 12.Godfrey LR, et al. In: Elwyn Simons: A Search for Origins. Fleagle JG, Gilbert CC, editors. New York: Springer; 2008. pp. 361–395. [Google Scholar]
- 13.Godfrey LR, Jungers WL, Wunderlich RE, Richmond BG. Reappraisal of the postcranium of Hadropithecus (Primates, Indroidea) Am J Phys Anthropol. 1997;103:529–556. doi: 10.1002/(SICI)1096-8644(199708)103:4<529::AID-AJPA9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 14.Godfrey LR, Jungers WL. In: The Primate Fossil Record. Hartwig W, editor. New York: Cambridge Univ Press; 2002. pp. 97–122. [Google Scholar]
- 15.Lemelin P, et al. New hand bones of Hadropithecus stenognathus: Implications for the paleobiology of the Archaeolemuridae. J Hum Evol. 2008;54:405–413. doi: 10.1016/j.jhevol.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Walker A, Ryan TM, Silcox MT, Simons E, Spoor F. The semicircular canal system and locomotion: the case of extinct lemuroids and lorisoids. Evol Anth. 2008;17:135–145. [Google Scholar]
- 17.Jolly CJ. Hadropithecus: A lemuroid small-object feeder. Man. 1970;5:620–626. [Google Scholar]
- 18.Iwamoto T, Dunbar RIM. Thermoregulation, habitat quality and the behavioral ecoogy of gelada baboons. J Anim Ecol. 1983;52:357–366. [Google Scholar]
- 19.Rafferty KL, Teaford MF, Jungers WL. Molar microwear of subfossil lemurs: Improving the resolution of dietary inferences. J Hum Evol. 2002;43:645–657. doi: 10.1006/jhev.2002.0592. [DOI] [PubMed] [Google Scholar]
- 20.Godfrey LR, et al. Dental use wear in extinct lemurs: Evidence of diet and niche differentiation. J Hum Evol. 2004;47:145–169. doi: 10.1016/j.jhevol.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Codron D, Lee-Thorp JA, Sponheimer M, de Ruiter D, Codron J. Inter- and intrahabitat dietary variability of chacma baboons (Papio ursinus) in South African savannas based on fecal delta C-13, delta N-15, and %N. Am J Phys Anthropol. 2006;129:204–214. doi: 10.1002/ajpa.20253. [DOI] [PubMed] [Google Scholar]
- 22.Codron J, et al. Taxonomic, anatomical, and spatio-temporal variations in the stable carbon and nitrogen isotopic compositions of plants from an African savanna. J Arch Sci. 2005;32:1757–1772. [Google Scholar]
- 23.Loudon JE, Whitelaw DC, Sponheimer M, Sauther ML, Cuozzo FP. Lemurs eating isotopes: A stable isotope analysis of ring-tailed lemurs (Lemur catta) and their menu at the Beza Mahafaly Special Reserve. Am J Phys Anthropol Suppl. 2008;46:142. [Google Scholar]
- 24.Lucas P, Constantino P, Wood B, Lawn B. Dental enamel as a dietary indicator in mammals. BioEssays. 2008;30:374–385. doi: 10.1002/bies.20729. [DOI] [PubMed] [Google Scholar]
- 25.Weber GW, Bookstein FL. Virtual Anthropology. Vienna: Springer; 2008. [Google Scholar]
- 26.Kirk EC. Visual influences on primate encephalization. J Hum Evol. 2006;51:76–90. doi: 10.1016/j.jhevol.2006.01.005. [DOI] [PubMed] [Google Scholar]