Abstract
The perisylvian region of the human cortex is known to play a major role in language processing. Especially the superior temporal cortex (STC) and the inferior frontal cortex (IFC) have been investigated with respect to their particular involvement in language comprehension. In the present research, the timing of recruitment of these language-related brain areas in both hemispheres was examined as a function of age using functional imaging data of 6-year-old children and adults with a special focus on blood oxygenation level dependent (BOLD) response time courses. The results show that children’s activation time courses differ from that of adults. First, children show an overall later peak of BOLD responses. Second, children’s IFC responds much later than their STC, while in adults the difference between both regions is less pronounced. Within the STC, both groups show similar regionally U-shaped activation patterns with fastest peaks in voxels at the STC’s mid-portion around Heschl’s gyrus and longer latencies in anterior and posterior directions, suggesting a coarsely similar information flow in adults and children in the temporal region. Finally, children in contrast to adults, display a temporal primacy of right over left hemispheric activation. The observed overall latency differences between children and adults are in line with the assumption of ongoing maturation in perisylvian brain regions and the connections between them. A functional perspective on BOLD timing argues for a developmental change from higher processing costs in children compared to adults due to slower and less automatic language processes, in particular those located in the IFC. The observed hemispheric differences are discussed in the context of developmental models assuming a high reliance on right-hemisphere-based suprasegmental information processing during language comprehension in childhood.
Introduction
By means of brain imaging methods, in particular functional magnetic resonance imaging (fMRI), we have progressively learned about the involvement of the perisylvian region of the human cortex in language processing and respective contributions of frontal, temporal, and parietal brain areas in different linguistic aspects such as syntax, semantics, and phonology (Friederici, 2002; Hickok and Poeppel, 2007). For auditory language perception, specialized left and right hemispheric involvement has been reported, with the left hemispheric (LH) perisylvian cortex supporting the processing of semantic and syntactic information (Friederici, 2002), and with the perisylvian cortex of the right hemisphere (RH) being responsible for processing prosodic information (Meyer et al., 2002; Zatorre et al., 2002). These experimental data suggest a model for adult language comprehension that assumes segmental information to be processed predominantly in LH and suprasegmental information to be processed primarily in RH (Friederici and Alter, 2004). A right hemispheric specialization similar to adults was reported already in young children, e.g. for the processing of prosodic information (Homae et al., 2006; Wartenburger et al., 2007). An increase in language lateralization to the left hemisphere, however, was observed with increasing age (Holland et al., 2001; Szaflarski et al., 2006).
A recent developmental fMRI study on syntactic and semantic processing during language comprehension found higher involvement of the perisylvian language areas in 6-year-old children compared to adults (Brauer and Friederici, 2007). Crucial areas of activation were primarily Broca’s area, its right-hemispheric homologue and the deep frontal operculum (FO) bilaterally in the inferior frontal cortex (IFC) as well as the superior temporal cortex (STC) bilaterally. In adults, IFC and STC were also involved, but IFC activation was limited to the left hemispheric FO, whereas activation of the more lateral part of IFC (Broca’s area) remained below threshold. Interestingly, adults showed differentiation between semantic and syntactic processes in the FO and along the STC, whereas children only showed such a functional differentiation in Broca’s area.
The present research extended on this initial work on children’s language comprehension by focusing on the time course of activation in language-related brain regions. Specifically, here we investigated temporal dynamics of the BOLD signal within brain areas involved in language comprehension. The time course of brain activation has been usually examined by means of event-related brain potentials (ERPs) or event-related fields (ERFs). While temporal information in ERP and ERF data is of high precision, spatial information is unfortunately rather imprecise. Conversely, very high spatial resolution about brain areas involved in cognitive processing can be gained from fMRI. As the underlying physiology also contains information about temporal dynamics of brain activation (Friston et al., 1995), the hemodynamic timing of functionally identified brain areas can also be obtained from fMRI data in addition to the spatial information.
Several methods have been developed to extract and investigate temporal information from hemodynamic brain responses. For example, spectral analysis using measures of coherence and phase of the BOLD signal have been administered to human brain data (Dehaene-Lambertz et al., 2006a; Müller et al., 2003; Sun et al., 2005). With these approaches, sequences of fMRI brain activation were separated at temporal resolutions down to about 100 milliseconds (Sigman et al., 2007). Also time-to-peak and other parameters have been extracted from the BOLD time course to describe its temporal behaviour (Bellgowan et al., 2003; Neumann et al., 2003; Thierry et al., 1999). Both measures of hemodynamic latency i.e., spectral phase shift and time-to-peak of the BOLD time course, were confirmed to highly correlate with each other, in particular for short stimulation times up to several seconds (Müller et al., 2005). Other approaches have employed independent component analysis (Duann et al., 2002) or nonlinear regression analysis (Kruggel and von Cramon, 2001) to model the BOLD response under specific assumptions.
It was shown that the time course of the BOLD response varies between different brain areas (Anemueller et al., 2006; Duann et al., 2002; Thierry et al., 1999). Moreover, the latency of the BOLD response can even be selectively affected in specific brain regions by cognitive demands such as verbal working memory load (Thierry et al., 2003), by stimulus repetition (Dehaene-Lambertz et al., 2006a), or lexical decision (Henson et al., 2002). While temporal activation (Wernicke’s area) appears earlier than inferior frontal activation (Broca’s area) in language comprehension, language production, in contrast, is characterized by an opposite pattern of peak activation with temporal primacy for Broca’s over Wernicke’s area (Heim and Friederici, 2003).
The present research on a comparison of children’s activation time courses to the adult time course pattern will provide a developmental perspective on temporal hemodynamics. In order to gain a more fine-grained insight into the temporal dynamics’ development of language-related brain recruitment in IFC and STC, we analyzed the time course of the BOLD response during sentence processing in these regions. Time-to-peak information was extracted from the hemodynamic response. The time-to-peak measure has been confirmed a very robust parameter along the BOLD time course (Neumann et al., 2003).
Materials and Methods
Participants and Material
The present study is a reanalysis of previously published data (Brauer and Friederici, 2007), this time investigating hemodynamic activation time courses. Data of 13 adults (7 female mean age 25.9 years, SD = 2.7) and 12 children (8 girls, mean age 6.2 years, SD = 0.7) were available. Parents of the children and the adult participants themselves gave written, informed consent. Children gave verbal assent for attendance. All children had normal intelligence and language skills and no known neurological or psychiatric disease or disorder or medical treatment affecting the central nervous system. None of the adult participants had any history of neurological or psychiatric disorder. All participants were right-handed German native speakers (Oldfield, 1971). The study was approved by the Research Ethics Committee of the University of Leipzig (Germany).
Stimulus material consisted of short sentences in active voice with age appropriate vocabulary. Items were spoken by a trained female native speaker in a well-pronounced, child-directed manner. All sentences were recorded and digitized at 44.1 kHz, 16 bit mono. They had an average length of about 2 seconds. For the adult sample, the session contained 200 trials plus 25 null events, in which the BOLD response was allowed to return to baseline state (Burock et al., 1998). For children, the session contained 120 trials plus 15 null events. Otherwise the procedure was the same as that used for adults. Trials were presented every 8 seconds in a single session. While listening to stimuli and during the entire measurement, participants could see an aquarium screensaver with fishes swimming calmly across the scene. Onset of every stimulus presentation relative to the beginning of the first scan was randomly jittered between 0, 500, 1,000, and 1,500 ms to get measurements at numerous time points along the BOLD signal curve, thus providing a higher resolution of the BOLD response (Miezin et al., 2000).
Scanning Parameters
For functional measurements, a gradient-echo EPI sequence was used (TE 30 ms, flip angle 90°, TR 2 s, bandwidth 100 kHz, matrix 64 × 64 voxels, FOV 192 mm, in-plane resolution 3 × 3 mm,) at 3 T (Siemens TRIO, Germany) for 20 slices (slice thickness 4 mm, 1 mm gap), covering a range of z = −40 mm to z = 60 mm from the AC-PC line. T1-weighted modified driven equilibrium Fourier transform (MDEFT) images (Ugurbil et al., 1993), matrix 256 × 256, TR 1.3 s, TE 7.4 ms, with a non slice-selective inversion pulse followed by a single excitation of each slice (Norris, 2000) were used for registration. For anatomical data, a T1-weighted 3D magnetization-prepared rapid gradient echo (MP-RAGE) sequence was obtained with magnetization preparation consisting of a non-selective inversion pulse (TI 650 ms, TR 1.3 s, snapshot FLASH 10 ms, TE 3.97 ms, angle 10 degrees, bandwidth 67 kHz, matrix 256 × 240, slab thickness 192 mm, sagittal orientation, spatial resolution 1 × 1 × 1.5 mm. To avoid aliasing, oversampling was performed in read direction (head-foot).
Data Analysis
For increased signal-to-noise ratio, no distinction between syntactic and semantic information processing was made. Rather, language stimulation in general was contrasted against resting baseline (null events) since our main interest was the temporal dynamics of the BOLD response in general language comprehension. Data processing was conducted with the LIPSIA software package (Lohmann et al., 2001) and included motion correction using three translational and three rotational parameters, slice time correction (cubic-spline-interpolation), highpass filtering (1/60 Hz), and spatial smoothing (4.24 mm full width at half maximum, FWHM). Motion correction was allowed up to 3 mm (one voxel). Three datasets of children were cut after 376, 460, and 532 of 540 repetitions for too much movement. Rotational and translational parameters of rigid linear registration were transformed to standard size by linear scaling (Talairach and Tournoux, 1988), followed by a nonlinear normalization (Thirion, 1998).
Statistical evaluation of functional activation was based on a general linear regression with pre-whitening (Worsley et al., 2002). Specifically, autocorrelation parameters were estimated from the least squares residuals using the Yule-Walker equations. These parameters were subsequently used to whiten both data and design matrix. Finally, the linear model was re-estimated using least squares on the whitened data to produce estimates of effects and their standard errors. The design matrix was generated with a synthetic hemodynamic response function (Friston et al., 1998; Josephs et al., 1997) and its first and second derivatives. Movement correction parameters and stimulus duration were included as regressors. For each participant, one contrast image was generated to represent the main effect of sentence presentation vs. baseline. Individual functional datasets were aligned with the stereotactic group reference space.
Statistical evaluation of BOLD time course was based on the following procedure. Individual fixed-effects z-maps at z > 2.33 (p < .01, uncorrected) were generated and used to mask individual preprocessed raw data. This was to guarantee that only reliably activated voxels would enter subsequent analysis where BOLD response information was obtained voxel-wisely from trial-averaged time courses for each subject by aligning the onsets for individual trials and averaging across these trials at a sampling rate of 5 Hz. Time points falling between measured data points due to jittering and the lower sampling rate for measuring were linearly interpolated from weighted values of their neighbors. Trial averages obtained for null-events were subtracted from critical trial averages. Subsequently, maximum amplitude (in percent signal change, peak maximum minus preceding minimum) and the corresponding time-to-peak measures were determined for every time course within a time range from 3 to 12 seconds as described by Neumann et al. (2003). This methodological approach of parameter extraction from trial averaged time courses relies on the assumption of stationarity and reproducibility of the hemodynamic response over trials. In cases where these assumption might not hold (e.g., habituation paradigms), there are alternative methods like model-based approaches for discriminating adaptation phenomenae (e.g., Marrelec et al., 2003).
Finally, time course parameters were averaged for both groups separately and entered in group maps. Further analyses investigated the perisylvian language region more closely. The region was subdivided into subclusters of activation to examine their contributions in more detail. The IFC was subdivided into Broca’s area and FO, the STC was subdivided along the y-axis in three subparts: anterior (ant STC), mid-portion (mid STC), and posterior STC (post STC). In order to acquire ROI time-to-peak values, voxel-wisely extracted time course information was averaged across subjects for each group and ROI, and obtained values were entered in repeated-measures GLM for statistical comparison.
Bonferroni correction for multiple comparisons of post-hoc analyses and Greenhouse-Geisser correction of degrees of freedom were applied as required (Greenhouse and Geisser, 1959).
Results
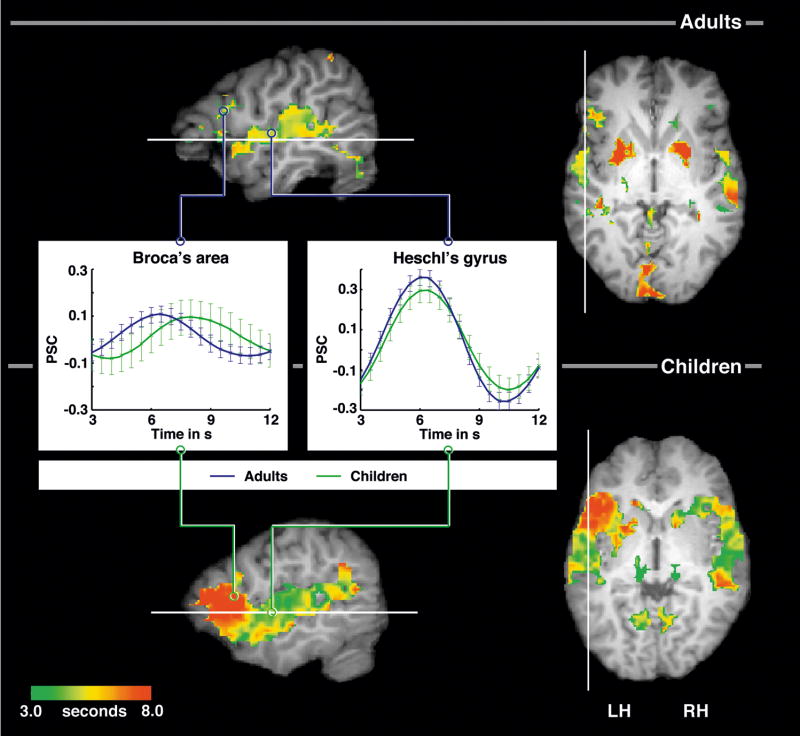
FMRI data on sentence comprehension in adults and children were analyzed with a focus on time courses of IFC and STC contributions to language processing in both groups. BOLD response parameters extracted voxel-wisely from individual preprocessed EPI maps were obtained. Only active voxels within IFC and STC were entered in subsequent analyses (see Figure 1).
Figure 1.
Temporal organization of cortical activation during sentence comprehension for adults and children in sagittal section (x = −50) and horizontal section (z = 2). Data are masked by random-effects activation maps at z > 2.33 and display a color coding for time-to-peak values in active voxels between 3.0 and 8.0 seconds. The lines indicate the cut for the corresponding section. Note the very late response in the inferior frontal cortex in children and their hemispheric differences in this region. Inserted diagrams demonstrate examples of BOLD responses to sentence comprehension in adults and children in Broca’s area (BA) and in Heschl’s gyrus (HG). Talairach coordinates BA: adults −49 17 17, children −49 12 11, HG: adults −52 −10 8, children −46 −12 2. BOLD response information is obtained voxel-wisely from preprocessed raw data. Crosses and standard error bars indicate measured data points (TR 2 s with onset jitter of 0.5 s). Line graphs illustrate subsequent interpolation (resolution 0.2 s).
We first tested contributions of the IFC in adults at the lower threshold. This preceding analysis should confirm a sufficient involvement of this area in adults, since strong activation within the IFC was found for both groups in the FO, but only for children in Broca’s area at a higher threshold in a previous analysis (Brauer and Friederici, 2007). Analysis revealed Broca’s area activation in adults with a maximum z-value of 2.80 (children: 3.88). To get further evidence for a lower but significant involvement of Broca’s area in adults, data were investigated for percent signal change (PSC) within the IFC. PSC values (incl. SD) for adults were 0.78 (0.23) in Broca’s area and 0.84 (0.24) in Broca’s homologue, 0.50 (0.10) in left FO and 0.53 (0.11) in right FO, for children 1.10 (0.47) in Broca and 0.97 (0.48) in Broca’s homologue, 0.70 (0.22) in left FO and 0.74 (0.33) in right FO. Data were investigated by a 2 × 2 × 2 repeated-measures GLM with between-subject factor Group (children, adults) and within-subject factors Area (Broca, FO) and Hemisphere (left, right). A significantly lower PSC of Broca’s area in adults would result in a Group × Area interaction. However, we found significant effects for Area, F(1,23) = 38.12, p < .001, and Group, F(1,23) = 4.63, p < .05, but no effect for hemisphere, F(1,23) < 1, and no significant interaction: Group × Area: F(1,23) < 1, Group × Hemisphere: F(1,23) = 1.54, p = .23, Area × Hemisphere: F(1,23) = 1.40, p = .25, Group × Area × Hemisphere: F(1,23) = 2.65, p = .12. Thus, children show higher PSC values than adults, and Broca’s area higher PSC values than the FO. The main effect for Area without a Group × Area interaction indicates the same tendency for IFC recruitment in children and adults.
In order to investigate temporal dynamics of language-related brain activation in the perisylvian region, we investigated BOLD response time-to-peak latencies more closely in inferior frontal cortex and superior temporal cortex. Time-to-peak information was obtained and statistically compared between groups and hemispheres for Broca’s area, FO, ant STC, mid STC, and post STC. Active voxels within these areas were entered in a 5 (Area) × 2 (Hemisphere) × 2 (Group) repeated-measures GLM. Analysis revealed a significant main effect for Group with time-to-peak mean values (incl. SD) of 5.8 s (0.4) for adults and 6.7 s (0.6) for children. There was also a significant main effect for Hemisphere, LH: 6.4 s (0.9), RH: 6.1 s (0.6), and a main effect for Area with fastest responses in the STC [mid STC: 5.7 s (0.6), ant STC: 6.2 s (0.8), post STC: 6.3 s (1.0)] and slower responses in the IFC [Broca: 6.6 s (0.9), FO: 6.6 s (1.0)]. Interactions were found for Group × Area and for Group × Hemisphere, and also the Group × Area × Hemisphere interaction yielded significance (see Figure 2 and Table 1). Consequently, post-hoc analyses were run for each level of factor Area with the following results. In the IFC, a Group main effect was found for Broca’s area with BOLD latencies of 6.0 s (0.5) for adults and 7.2 s (0.8) for children. Also for the FO area, analysis yielded a significant Group effect [adults: 6.0 s (0.6), children: 7.2 s (0.9)], and, moreover, a Group × Hemisphere interaction, based on a significant hemispheric difference in children [LH: 7.8 s (1.3), RH: 6.6 s (0.7)], F(1,11) = 8.37, p < .05, while there was no such hemispheric effect in adults [LH: 5.8 s (0.6), RH: 6.1 s (0.8), F(1,12) = 2.77, p = .37]. In the STC, for mid STC and post STC, a significant Group effect was observed: mid STC 5.4 s (0.4) for adults and 6.0 s (0.6) for children, post STC 5.7 s (0.4) for adults and 6.8 s (1.2) for children, while no significant effect was observed for ant STC (see Table 2). A post-hoc analysis of the overall Group × Hemisphere interaction from the initial 5 × 2 × 2 analysis revealed this effect to rely on hemispheric differences in children [LH: 7.0 s (0.8), RH: 6.4 s (0.6), F(1,11) = 8.27, p < .05], not in adults [LH: 5.8 s (0.5), RH: 5.8 s (0.4), F(1,12) < 1].
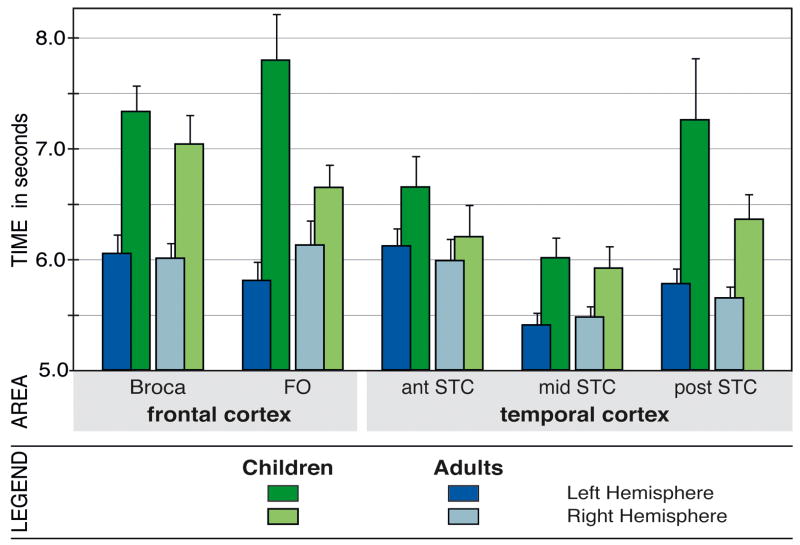
Figure 2.
Bar graphs for time-to-peak latencies in seconds (+ standard error) in perisylvian language areas for children and adults within the left and the right hemisphere. In both groups, data show fastest responses in mid STC and shorter latencies in temporal cortex than in frontal cortex in both groups. However, children show overall longer latencies than adults and their fronto-temporal latency differences are much more pronounced than the fronto-temporal differences in adults. Furthermore, children, but not adults, demonstrate hemispheric differences with slower left than right hemispheric activation (cf. Figure 1). Broca = Broca’s area, FO = frontal operculum, STC = superior temporal cortex, ant = anterior, mid = mid-portion, post = posterior.
Table 1. Results of a 5 × 2 × 2 repeated-measures analysis for BOLD time-to-peak.
Repeated-measures GLM results for BOLD time-to-peak. Factors are between-subject factor Group (adults, children) and within-subject factors Area (Broca, FO, ant STC, mid STC, post STC) and Hemisphere (left, right).
Broca = Broca’s area, FO = frontal operculum, STC = superior temporal cortex, ant = anterior, mid = mid-portion, post = posterior.
| Effect | df | F-value | p-value |
|---|---|---|---|
| Group | 1,23 | 17.89 | p < .001 |
| Area | 4,92 | 10.47 | p < .001 |
| Hemisphere | 1,23 | 7.67 | p < .05 |
| Group × Area | 4,92 | 3.15 | p < .05 |
| Group × Hemisphere | 1,23 | 8.63 | p < .01 |
| Area × Hemisphere | 4,92 | 1.90 | p = .16 |
| Group × Area × Hemisphere | 4,92 | 3.56 | p < .05 |
Table 2. Post-hoc repeated-measures analysis of BOLD time-to-peak for each level of factor Area.
Repeated-measures GLM results for BOLD time-to-peak, post-hoc analysis for factor Area. There is a significant Group effect in Broca, FO, mid STC, and post STC, and a significant Group × Hemisphere interaction in the FO.
Broca = Broca’s area, FO = frontal operculum, STC = superior temporal cortex, ant = anterior, mid = mid-portion, post = posterior.
| Broca | FO | ant STC | mid STC | post STC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | df | F-value | p-value | F-value | p-value | F-value | p-value | F-value | p-value | F-value | p-value |
| Group | 1,23 | 19.55 | p < .001 | 16.81 | p < .001 | 1.51 | p = .46 | 6.75 | p < .05 | 9.47 | p < .01 |
| Hemisphere | 1,23 | 1.94 | p = .36 | 3.72 | p = .13 | 5.48 | p = .06 | < 1 | 4.90 | p = .07 | |
| Group × Hemisphere | 1,23 | 1.07 | p = .62 | 11.65 | p < .01 | 1.61 | p = .43 | 1.80 | p = .39 | 2.70 | p = .23 |
Results indicated a robust main effect of Group in the initial 5 × 2 × 2 GLM, confirmed by the same effect in nearly all areas in the post-hoc analysis. That indicates overall longer BOLD latencies in children than in adults. The Area effect in the initial analysis is based on an equivalent tendency in both groups with shortest latencies in the mid STC and longest latencies in the IFC. While in post-hoc analyses in the FO area of the IFC a significant Group × Hemisphere interaction was found, no such interaction was observed in STC areas. The overall main effect of Hemisphere was confirmed in the children’s FO, and for both groups marginally (p < .10) in the STC (ant and post).
In a separate region analysis, the timing of inferior frontal and superior temporal contributions was contrasted. For that purpose, a 2 × 2 × 2 repeated-measures GLM was conducted with between-subject factor Group (children, adults) and within-subject factors Area (IFC, STC) and Hemisphere (left, right). Statistical comparison yielded significant main effects for all three factors as well as significant Group × Area, Group × Hemisphere, and Group × Area × Hemisphere interactions (see Table 3A). Interactions were further investigated by a follow-up analysis comparing each level of factor Group separately. Results revealed an Area main effect for adults with IFC: 6.0 s (0.5), STC: 5.7 s (0.4). For children, too, an Area main effect was observed, IFC: 7.2 s (0.8), STC: 6.4 s (0.6), and also the Hemisphere main effect in children was confirmed, LH: 7.0 s (0.8), RH: 6.4 s (0.6) (see Table 3B). Theses results reveal the differences between children and adults with respect to the timing of inferior frontal and superior temporal contributions, as pointed out by the Group × Area interaction. Although both groups demonstrate later IFC than STC activation, this effect size was only moderate in adults (ηp2 = .41), while the same effect was large in children (ηp2 = .65).
Table 3.
Contrast of IFC vs. STC BOLD time-to-peak measures (Table 3A). Factors are between-subject factor Group (adults, children) and within-subject factors Area (IFC, STC) and Hemisphere (left, right). The Group × Area and the Group × Hemisphere interactions reveal distinct patterns of frontal vs. temporal activation time courses for both groups. A follow-up analysis for both Groups separately reveals the Area effect for each Group, although lower effect size in adults (ηp2 = .41) than in children (ηp2 = .65). The Hemisphere effect is based on a hemispheric distinction in children, but not in adults (Table 3B).
IFC = inferior frontal cortex, STC = superior temporal cortex, LH = left hemisphere, RH = right hemisphere.
| Table 3A: BOLD time-to-peak contrast for IFC vs. STC areas | |||
|---|---|---|---|
| Effect | df | F-value | p-value |
| Group | 1,23 | 18.98 | p < .001 |
| Area | 1,23 | 29.52 | p < .001 |
| Hemisphere | 1,23 | 7.21 | p < .05 |
| Group × Area | 1,23 | 7.61 | p < .05 |
| Group × Hemisphere | 1,23 | 9.24 | p < .01 |
| Area × Hemisphere | 1,23 | < 1 | |
| Group × Area × Hemisphere | 1,23 | 5.32 | p < .05 |
| Table 3B. Post-hoc analysis for IFC vs. STC separated by levels of factor Group | |||
| Adults | |||
|
| |||
| Effect | df | F-value | p-value |
|
| |||
| Area | 1,12 | 8.56 | p < .05 |
| Hemisphere | 1,12 | < 1 | |
| Area × Hemisphere | 1,12 | 3.00 | p = .22 |
| Children | |||
|
| |||
| Effect | df | F-value | p-value |
|
| |||
| Area | 1,11 | 19.96 | p < .05 |
| Hemisphere | 1,11 | 8.39 | p < .05 |
| Area × Hemisphere | 1,11 | 2.42 | p = .30 |
Discussion
This study examined BOLD response properties in perisylvian language areas in 6-year-old children and adults. In both groups, similar brain regions in inferior frontal and superior temporal cortices were activated bilaterally. Analysis of BOLD amplitudes in Broca’s area and FO revealed reliable involvement of Broca’s area in adults albeit observable only at a lower threshold than in a previous analysis (Brauer and Friederici, 2007).
For BOLD response latencies a systematic progression was observed along the STC with fastest BOLD responses in the mid-portion around Heschl’s gyrus and longer latencies in anterior and posterior directions, suggesting initial processing of sensory information in primary auditory cortex and later involvement of anterior and posterior STC and the IFC. This pattern was in general equivalent for both groups and represents a finding that is in line with earlier studies in infants (Dehaene-Lambertz et al., 2006b) and adults (Dehaene-Lambertz et al., 2006a; Thierry et al., 1999). A direct comparison between age groups revealed that children showed overall longer BOLD latencies than adults. We excluded a possible confound of experiment length on the group differences by an additional analysis. Since the experimental session for children was shorter than for adults (540 vs. 900 scanning repetitions), the effect for BOLD latencies could have been based on the longer experiment in adults. We simulated a shorter experiment in adults by truncating the adult datasets after 540 repetitions. Accordingly, we obtained a short and a long version of the experiment for adult participants. A repeated-measures GLM for these two versions yielded no effect of factor Length [F(1,12) = 1.20, p = .29] and no interaction of Length with any other factor: Length × Area [F(4,48) < 1], Length × Hemisphere [F(1,12) < 1], Length × Area × Hemisphere [F(4,48) < 1]. Hence, length of experiment cannot explain the observed group differences.
Besides the overall longer BOLD latencies in children compared to adults, time-to-peak latencies were observed to be longer in IFC than in STC for both groups. However, in children this effect was much more pronounced, as pointed out by the significant interaction in the region analysis. But children did not only show a stronger temporal-frontal regional effect, they, moreover, demonstrated a significant effect across hemispheres with slower left and faster right-hemispheric BOLD responses, a finding which was absent in adults. Particularly these group by area and group by hemisphere effects require some more profound discussion.
In two previous studies, BOLD timing properties in perisylvian areas during sentence comprehension were reported for adults and for 3-month-old infants, respectively. These studies suggested similar patterns in the temporal organizations of superior temporal and inferior frontal cortices when considering the infants’ data (Dehaene-Lambertz et al., 2006b) and those referred for adults (Dehaene-Lambertz et al., 2006a). They did not report a quantitative comparison between infants and adults, but their conclusion of a qualitatively similar temporal organization in the developing and the mature brain seems to confirm the results of the present study. However, in absence of a direct comparison between infants and adults, an evaluation of temporal dynamics between the two groups in these areas remains difficult. The present data of 6-year-old children and adults rather argue for a developmental effect of these contributions to sentence comprehension with later BOLD responses in inferior frontal areas and a hemispheric effect with earlier right than left hemispheric hemodynamic responses in the developing language system.
Differences between children and adults in the present study might reflect processing differences between age groups in a way that higher cognitive processing demand may cause delayed BOLD responses. The present study cannot yield such conclusions by itself. However, studies in adults have shown that the hemodynamic timing of brain responses can be influenced by experimental manipulation (Dehaene-Lambertz et al., 2006a; Heim and Friederici, 2003; Henson et al., 2002; Thierry et al., 2003). For instance, the latency of BOLD peaks for language processing has been shown to be delayed by additional verbal working memory requirements (Thierry et al., 2003). They demonstrated that evoked hemodynamic responses in inferior prefrontal cortex including Broca’s area depended on experimental manipulation with varying demands for verbal working memory. The finding that in this study no such sensitivity of BOLD time course was observed in the superior temporal gyrus, argues for region-specific effects based on varying cognitive demands. Accordingly, our data suggest higher cognitive processing demands for the processing of sentences in the developing brain as opposed to the adult brain. More automatic and thereby faster language processing in the mature as compared to the developing brain might account for the differences in BOLD time courses in IFC between children and adults.
The interpretation of quantitatively different processes in language processing in adults and children is supported by results from electrophysiological studies. ERP brain responses related to sentence comprehension processes have been reported to be delayed in children compared to adults (Hahne et al., 2004; Holcomb et al., 1992; Oberecker et al., 2005). In the semantic domain, this delay has been interpreted to reflect increasing demands on contextual integration processes (Holcomb et al., 1992), and in the syntactic domain component delay has been interpreted to reflect slower processes. The absence of a particular ERP component indexing automatic syntactic processes has been argued to indicate that the automaticity of syntactic processes only develops slowly during childhood (Hahne et al., 2004; Oberecker and Friederici, 2006; Oberecker et al., 2005).
We observed equal overall hemodynamic time-to-peak values in adults for both hemispheres, but smaller values for RH in children, based particularly on the right FO. The right-hemispheric FO has been shown to be sensitive to suprasegmental, prosodic information in functional imaging studies in adults (Dehaene-Lambertz et al., 2006a; Friederici and Alter, 2004; Meyer et al., 2004). A right hemispheric involvement for prosodic processes was also reported for 4-year-old children (Wartenburger et al., 2007) and for infants (Homae et al., 2006), both by means of near-infrared spectroscopy (NIRS). Moreover, ERP data have demonstrated that in adults prosodic information influences syntactic parsing very fast, that is in a very early phase during speech comprehension (Eckstein and Friederici, 2006) and that the brain’s sensitivity to prosodic features is present not only in adults (Pannekamp et al., 2005), but also in infants (Pannekamp et al., 2006).
Psycholinguistic studies in adults (Marslen-Wilson et al., 1992; Warren et al., 1995) have provided evidence for an interaction of prosodic and syntactic processes during auditory language comprehension (Frazier et al., 2006), and psycholinguistic models of language acquisition state a strong reliance on prosodic information during early language processing (Weissenborn and Höhle, 2001). The shorter right than left BOLD latencies for children in our study seem to match these electrophysiological data and, moreover, are consistent with the psycholinguistic models. The present data indicate a temporal hemodynamic primacy of the right hemisphere in the developing brain, particularly the right FO, possibly reflecting the intense use of prosodic information during language processing.
However, a direct comparison of hemodynamic and electrophysiological event-related responses should be interpreted with caution. Although the BOLD contrast mechanism is considered to reflect neural responses to a stimulus (Logothetis et al., 2001), the neurovascular interrelation of delay in neural activity and temporal properties of hemodynamic processes is in need for further clarification. It is still an open question whether observed BOLD response latency differences reflect a hemodynamic or neuronal origin or a synthesis of both. Hemodynamic response timing may reflect the timing of neuronal activity, but the inverse problem regarding inferences from hemodynamic responses to underlying neural activity only starts to be addressed (Buckner, 2003).
On the basis of the present study, it is up to now not possible to exactly evaluate to what extent observed differences in hemodynamic timing between adults and children are grounded on differences in local vasculature and on differences in functional recruitment of involved brain areas. A factor to be considered for an interpretation of the present findings might also be the potential influence of cerebral blood flow (CBF). The BOLD signal reflects changes in CBF relative to changes in cerebral metabolic rate of oxygen (CMRO2) (Buxton et al., 2004). By implementing a vascular model of the hemodynamic response, Vazquez et al. (2006) have suggested that changes in baseline CBF might influence latency and amplitude parameters of the BOLD signal. Concerning development, global cortical CBF was shown to increase during early childhood, peaking at about age 5 to 6, and then to decline, reaching an adult level in late adolescence (Chiron et al., 1992; Takahashi et al., 1999). Moreover, developmental changes in regional CBF were argued to be related to cognitive development and higher order functions such as language (Chiron et al., 1992; Devous et al., 2006). Thus, a potential influence of CBF differences between adults and children is conceivable to contribute to the present findings. However, there are still too many open questions in our current understanding of CBF development and its relation to evoked BOLD responses to agree upon robust conclusions at present.
In addition to functional and physiological aspects of the observed hemodynamic differences between adults and children, structural aspects of brain maturation must be considered. Our observation of overall longer BOLD latencies in children agrees with the assumption of ongoing maturational changes within language relevant brain areas and the structural connections between them. Regarding the developmental courses of white matter myelination in language-related temporal and frontal brain regions, Pujol et al. (2006) described temporal and frontal regions to coincide during rather early stages of maturation. The frontal cortex, however, is among the last brain regions to fully mature (Sowell et al., 1999). Structural maturation of white matter tracts in those fronto-temporal pathways which support language functions is even reported to continue until late childhood and adolescence (Paus et al., 1999). Relations between the maturation of brain structure and cognitive functions have been reported for gray and white matter. Nagy et al. (2004), for example, have shown that cognitive functions are related to maturation of white matter for children older than 8 years of age. Changes in gray matter maturation are known to continue until adulthood (Toga et al., 2006) and are correlated with changes in cognitive abilities such as vocabulary (Sowell et al., 2004). The relationship between maturation of brain structure and development of cognitive function so far, however, is correlative only and has to be investigated more thoroughly before specific causal inferences from synchronous brain maturation and progress in cognitive functions can be drawn (Aslin and Schlaggar, 2006). Nonetheless, maturation of gray and white matter must be considered as one aspect in the explanation for the apparent changes of BOLD time courses during development, even though the precise impact of brain maturation on the present results remains an open issue.
Taken together, a combination of neurophysiological and structural factors might account for differences in the temporal dynamics of brain responses between children and adults as it was observed in the present study. Moreover, a functional account can help to better understand the present findings. A possible scenario regarding functional and structural contributions to the development of language comprehension might be that the overall time course differences of hemodynamic responses between adults and children exist mainly due to ongoing maturational changes in children, whereas specific age differences between particular brain areas might be mainly based on differences in functional processing with structural properties contributing less. A case of almost purely functional influences might be the hemispheric age differences which most likely results from different processing strategies in children and adults with children relying more on right hemispheric prosodic processes than adults. In general, this might suggest that as long as children’s brains do not possess mature structural means, they need to compensate that disadvantage by strategy and/or effort. Progressing with further brain development (through maturation and experience), more effective information transmission and processing become possible.
Conclusion
The present study demonstrated distinct temporal dynamics of the BOLD response in the perisylvian language cortex for 6-year-old children and adults during language comprehension. Children’s BOLD responses showed overall longer latencies when compared to adults. Moreover, a temporal primacy of right over left hemispheric activation was found, especially for the children’s FO. While in adults, inferior frontal activation showed peak latencies later than but close to superior temporal activation, children’s IFC activation peaked much later than STC activation. These latency differences between children and adults in the functional BOLD response during language comprehension are in line with our current understanding of maturational changes in language-related brain areas and the structural connections between them. The data also support the view that developmental changes evolve from higher processing costs in the developing brain to faster and more automatic language processing in the mature brain.
Acknowledgments
JN’s work is supported by the NIH (Grant R01MH74457).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anemueller J, Duann JR, Sejnowski TJ, Makeig S. Spatio-temporal dynamics in fMRI recordings revealed with complex independent component analysis. Neurocomputing. 2006;69:1502–1512. doi: 10.1016/j.neucom.2005.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslin RN, Schlaggar BL. Is myelination the precipitating neural event for language development in infants and toddlers? Neurology. 2006;66:304–305. doi: 10.1212/01.wnl.0000201189.32928.fb. [DOI] [PubMed] [Google Scholar]
- Bellgowan PSF, Saad ZS, Bandettini PA. Understanding neural system dynamics through task modulation and measurement of functional MRI amplitude, latency, and width. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1415–1419. doi: 10.1073/pnas.0337747100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer J, Friederici AD. Functional Neural Networks of Semantic and Syntactic Processes in the Developing Brain. Journal of Cognitive Neuroscience. 2007;19:1609–1623. doi: 10.1162/jocn.2007.19.10.1609. [DOI] [PubMed] [Google Scholar]
- Buckner RL. The hemodynamic inverse problem: Making inferences about neural activity from measured MRI signals. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2177–2179. doi: 10.1073/pnas.0630492100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. NeuroImage. 2004;23:S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Chiron C, Raynaud C, Maziere B, Zilbovicius M, Laflamme L, Masure MC, Dulac O, Bourguignon M, Syrota A. Changes in Regional Cerebral Blood Flow During Brain Maturation in Children and Adolescents. J Nucl Med. 1992;33:696–703. [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Anton JL, Campagne A, Ciuciu P, Dehaene GP, Denghien I, Jobert A, LeBihan D, Sigman M, Pallier C, Poline JB. Functional segregation of cortical language areas by sentence repetition. Human Brain Mapping. 2006a;27:360–371. doi: 10.1002/hbm.20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Hertz-Pannier L, Dubois J, Meriaux S, Roche A, Sigman M, Dehaene S. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proceedings of the National Academy of Sciences of the United States of America. 2006b;103:14240–14245. doi: 10.1073/pnas.0606302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devous MD, Sr, Altuna D, Furl N, Cooper W, Gabbert G, Ngai WT, Chiu S, Scott JM, III, Harris TS, Payne JK, Tobey EA. Maturation of Speech and Language Functional Neuroanatomy in Pediatric Normal Controls. J Speech Lang Hear Res. 2006;49:856–866. doi: 10.1044/1092-4388(2006/061). [DOI] [PubMed] [Google Scholar]
- Duann JR, Jung TP, Kuo WJ, Yeh TC, Makeig S, Hsieh JC, Sejnowski TJ. Single-trial variability in event-related BOLD signals. NeuroImage. 2002;15:823–835. doi: 10.1006/nimg.2001.1049. [DOI] [PubMed] [Google Scholar]
- Eckstein K, Friederici AD. It’s early: Event-related potential evidence for initial interaction of syntax and prosody in speech comprehension. Journal of Cognitive Neuroscience. 2006;18:1696–1711. doi: 10.1162/jocn.2006.18.10.1696. [DOI] [PubMed] [Google Scholar]
- Frazier L, Carlson K, Clifton C. Prosodic phrasing is central to language comprehension. Trends in Cognitive Sciences. 2006;10:244–249. doi: 10.1016/j.tics.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Towards a neural basis of auditory sentence processing. Trends in Cognitive Sciences. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Alter K. Lateralization of auditory language functions: A dynamic dual pathway model. Brain and Language. 2004;89:267–276. doi: 10.1016/S0093-934X(03)00351-1. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. NeuroImage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RSJ. Characterizing Evoked Hemodynamics with fMRI. NeuroImage. 1995;2:157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Hahne A, Eckstein K, Friederici AD. Brain signatures of syntactic and semantic processes during children’s language development. Journal of Cognitive Neuroscience. 2004;16:1302–1318. doi: 10.1162/0898929041920504. [DOI] [PubMed] [Google Scholar]
- Heim SCA, Friederici AD. Phonological processing in language production: time course of brain activity. Neuroreport. 2003;14:2031–2033. doi: 10.1097/00001756-200311140-00005. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: Application to words versus nonwords and initial versus repeated face presentations. NeuroImage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Opinion - The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ, Coffey SA, Neville HJ. Visual and auditory sentence processing: A developmental analysis using event-related brain potentials. Developmental Neuropsychology. 1992;8:203–241. [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball JWS. Normal fMRI Brain Activation Patterns in Children Performing a Verb Generation Task. NeuroImage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Nakano T, Asakawa K, Taga G. The right hemisphere of sleeping infant perceives sentential prosody. Neuroscience Research. 2006;54:276–280. doi: 10.1016/j.neures.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Josephs O, Turner R, Friston K. Event-related fMRI. Human Brain Mapping. 1997;5:243–248. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kruggel F, von Cramon DY. Nonlinear regression analysis of the hemodynamic response in functional MRI. Pattern Recognition Letters. 2001;22:1247–1252. [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lohmann G, Müller K, Bosch V, Mentzel H, Hessler S, Chen L, Zysset S, von Cramon DY. LIPSIA - a new software system for the evaluation of functional magnetic resonance images of the human brain. Computerized Medical Imaging and Graphics. 2001;25:449–457. doi: 10.1016/s0895-6111(01)00008-8. [DOI] [PubMed] [Google Scholar]
- Marrelec G, Benali H, Ciuciu P, Pelegrini-Issac M, Poline JB. Robust Bayesian estimation of the Hemodynamic Response Function in event-related BOLD fMRI using basic physiological information. Human Brain Mapping. 2003;19:1–17. doi: 10.1002/hbm.10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marslen-Wilson WD, Tyler LK, Warren P, Grenier P, Lee CS. Prosodic effects in minimal attachment. Quarterly Journal of Experimental Psychology. 1992;45:73–87. [Google Scholar]
- Meyer M, Alter K, Friederici AD, Lohmann G, von Cramon DY. FMRI reveals brain regions mediating slow prosodic modulations in spoken sentences. Human Brain Mapping. 2002;17:73–88. doi: 10.1002/hbm.10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Steinhauer K, Alter K, Friederici AD, von Cramon DY. Brain activity varies with modulation of dynamic pitch variance in sentence melody. Brain & Language. 2004;89:277–289. doi: 10.1016/S0093-934X(03)00350-X. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. NeuroImage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Müller K, Mildner T, Lohmann G, von Cramon DY. Investigating the stimulus-dependent temporal dynamics of the BOLD signal using spectral methods. Journal of Magnetic Resonance Imaging. 2003;17:375–382. doi: 10.1002/jmri.10268. [DOI] [PubMed] [Google Scholar]
- Müller K, Neumann J, Lohmann G, Mildner T, von Cramon DY. The correlation between blood oxygenation level-dependent signal strength and latency. Journal of Magnetic Resonance Imaging. 2005;21:489–494. doi: 10.1002/jmri.20271. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of White Matter is Associated with the Development of Cognitive Functions during Childhood. J Cogn Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Neumann J, Lohmann G, Zysset S, von Cramon DY. Within-subject variability of BOLD response dynamics. NeuroImage. 2003;19:784–796. doi: 10.1016/s1053-8119(03)00177-0. [DOI] [PubMed] [Google Scholar]
- Norris DG. Reduced power multislice MDEFT imaging. JMRI-Journal of Magnetic Resonance Imaging. 2000;11:445–451. doi: 10.1002/(sici)1522-2586(200004)11:4<445::aid-jmri13>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Oberecker R, Friederici AD. Syntactic event-related potential components in 24-month-olds’ sentence comprehension. Neuroreport. 2006;17:1017–1021. doi: 10.1097/01.wnr.0000223397.12694.9a. [DOI] [PubMed] [Google Scholar]
- Oberecker R, Friedrich M, Friederici AD. Neural correlates of syntactic processing in two-year-olds. Journal of Cognitive Neuroscience. 2005;17:1667–1678. doi: 10.1162/089892905774597236. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pannekamp A, Toepel U, Alter K, Hahne A, Friederici AD. Prosody-driven sentence processing: An event-related brain potential study. Journal of Cognitive Neuroscience. 2005;17:407–421. doi: 10.1162/0898929053279450. [DOI] [PubMed] [Google Scholar]
- Pannekamp A, Weber C, Friederici AD. Prosodic processing at the sentence level in infants. Neuroreport. 2006;17:675–678. doi: 10.1097/00001756-200604240-00024. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Ortiz H, Sebastian-Galles N, Losilla JM, Deus J. Myelination of language-related areas in the developing brain. Neurology. 2006;66:339–343. doi: 10.1212/01.wnl.0000201049.66073.8d. [DOI] [PubMed] [Google Scholar]
- Sigman M, Jobert A, LeBihan D, Dehaene S. Parsing a sequence of brain activations at psychological times using fMRI. NeuroImage. 2007;35:655–668. doi: 10.1016/j.neuroimage.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FT, Miller LM, D’Esposito M. Measuring temporal dynamics of functional networks using phase spectrum of fMRI data. NeuroImage. 2005;28:227–237. doi: 10.1016/j.neuroimage.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Human Brain Mapping. 2006;27:202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Shirane R, Sato S, Yoshimoto T. Developmental changes of cerebral blood flow and oxygen metabolism in children. American Journal of Neuroradiology. 1999;20:917–922. [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Thierry G, Boulanouar K, Kherif F, Ranjeva JP, Demonet JF. Temporal sorting of neural components underlying phonological processing. Neuroreport. 1999;10:2599–2603. doi: 10.1097/00001756-199908200-00029. [DOI] [PubMed] [Google Scholar]
- Thierry G, Ibarrola D, Demonet JF, Cardebat D. Demand on verbal working memory delays haemodynamic response in the inferior prefrontal cortex. Human Brain Mapping. 2003;19:37–46. doi: 10.1002/hbm.10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirion JP. Image matching as a diffusion process: an analogy with Maxwell’s demons. Medical Image Analysis. 1998;2:243–260. doi: 10.1016/s1361-8415(98)80022-4. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neurosciences. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K, Garwood M, Ellermann J, Hendrich K, Hinke R, Hu XP, Kim SG, Menon R, Merkle H, Ogawa S, Salmi R. Imaging at high magnetic fields -initial experiences at 4-t. Magnetic Resonance Quarterly. 1993;9:259–277. [PubMed] [Google Scholar]
- Vazquez AL, Cohen ER, Gulani V, Hernandez-Garcia L, Zheng Y, Lee GR, Kim SG, Grotberg JB, Noll DC. Vascular dynamics and BOLD fMRI: CBF level effects and analysis considerations. NeuroImage. 2006;32:1642–1655. doi: 10.1016/j.neuroimage.2006.04.195. [DOI] [PubMed] [Google Scholar]
- Warren P, Grabe E, Nolan F. Prosody, phonology, and parsing in closure ambiguities. Language and Cognitive Processes. 1995;10:457–486. [Google Scholar]
- Wartenburger I, Steinbrink J, Telkemeyer S, Friedrich M, Friederici AD, Obrig H. The processing of prosody: Evidence of interhemispheric specialization at the age of four. NeuroImage. 2007;34:416–425. doi: 10.1016/j.neuroimage.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Weissenborn J, Höhle B. Approaches to Bootstrapping: Phonological, Lexical, Syntactic, and Neurophysiological Aspects of Early Language Acquisition. Benjamins; Amsterdam: 2001. [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC. A General Statistical Analysis for fMRI Data. NeuroImage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. Trends in Cognitive Sciences. 2002;6:37–46. doi: 10.1016/s1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]