Abstract
The polarization of axon and dendrites underlies the ability of neurons to integrate and transmit information in the brain. Important progress has been made towards the identification of the molecular mechanisms regulating neuronal polarization using primarily in vitro approaches such as dissociated culture of rodent hippocampal neurons. The predominant view emerging from this paradigm is that neuronal polarization is initiated by intrinsic activation of signaling pathways underlying the initial break in neuronal symmetry that precedes the future asymmetric growth of the axon. Recent evidence shows that (i) axon-dendrite polarization is specified when neurons engage migration in vivo, (ii) a kinase pathway defined by LKB1and SAD-kinases (Par4/Par1 dyad) is required for proper neuronal polarization in vivo and that (iii) extracellular cues can play an instructive role during neuronal polarization. Here, we review some of these recent results and highlight future challenges in the field including the determination of how extracellular cues control intracellular responses underlying neuronal polarization in vivo.
Introduction
Neurons are highly polarized cells presenting two molecularly and functionally distinct compartments emerging from the cell body: a single axon and multiple dendrites. The specification of axon and dendrites during neuronal polarization is an essential step underlying the proper transfer of synaptic information in the adult central nervous system. Over the past three decades, many studies have explored the cellular and molecular mechanisms underlying neuronal polarization using mostly an in vitro model introduced by Gary Banker two decades ago [1–3]. In this model, rodent hippocampal or cortical neurons are dissociated and cultured on a two-dimensional substrate. Quite remarkably, these neurons can polarize in the absence of any relevant extracellular cues to generate a single axon and multiple dendrites sharing several of the molecular attributes characterizing axon and dendrites in vivo [1]. In the past decade, these paradigm has been used extensively to identify proteins controlling the establishment of neuronal polarity including multiple kinases, phosphatases, small GTPases, microtubule-associated proteins, and scaffolding proteins [4–15• and 16]. Current challenges include determining which of these proteins play major roles in vivo and how they link extracellular determinants to intracellular effectors of neuronal polarity.
Axon-dendrite polarity is initiated during neuronal migration in mammals
Examination of axon-dendrite polarization in vivo revealed that this aspect of neuronal polarity is specified early on when neurons engage in radial migration through polarization of their leading and trailing process. Pioneering work by Pasko Rakic using Golgi staining and electron microscope (EM) serial-reconstruction of the morphology of migrating cortical neurons in primates revealed that axons emerge from their trailing process as they translocate [17]. This was confirmed by retrograde axon tracing showing that in primates, the cell bodies of a substantial number of callosal pyramidal neurons already have an axon in the contra-lateral hemisphere as their cell body translocates radially [18].
More recent investigation of the morphological changes characterizing migrating cortical neurons using time-lapse confocal microscopy confirmed that neurons go through a transient ‘multipolar’ phase when multiple neurites rapidly emerge from the cell body in all directions before adopting a classical unipolar morphology characterizing migrating neurons with a single leading process directed towards the cortical plate (Figure 1A) [19,20]. As neurons translocate through the intermediate zone of the cortex, the trailing process of migrating cortical neurons emerges from the ventral part of the cell body and extends rapidly to generate the axon [19–21••].
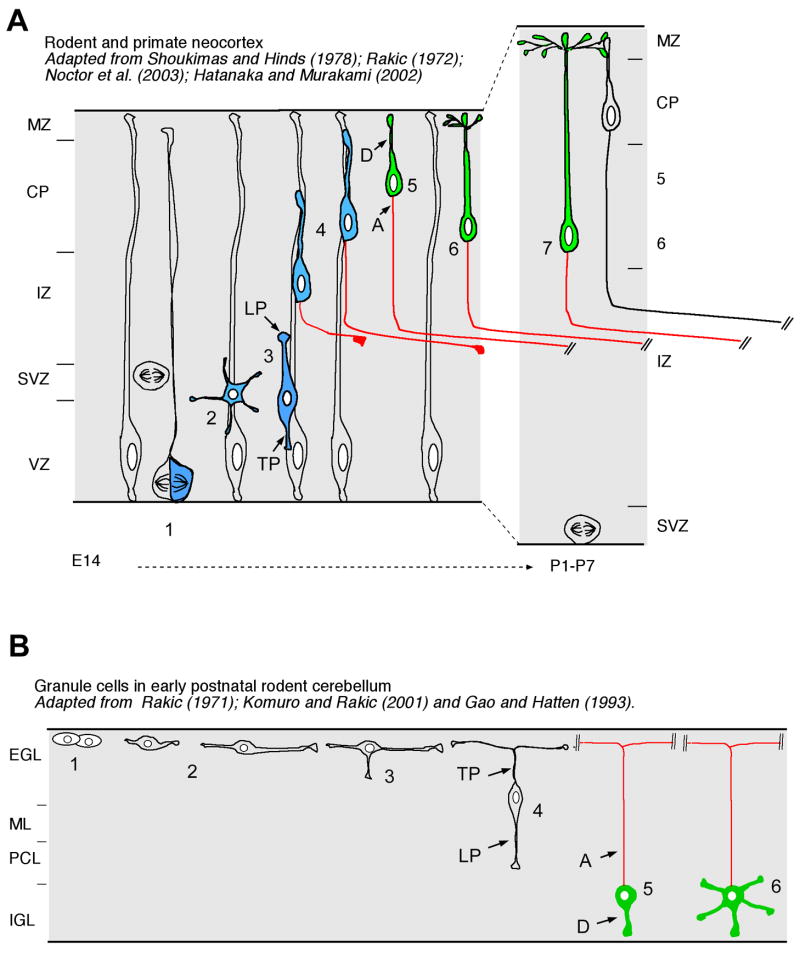
Figure 1. Cellular mechanisms underlying axon-dendrite polarization in vivo.
(A) In the cerebral cortex, axon/dendrite polarization occurs when neurons engage in radial migration in the intermediate zone [19–21]. Neurons are produced either from asymmetric ventricular division of radial glial progenitors in the ventricular zone (VZ, step 1) and/or neurogenic symmetric abventricular division of transient amplifying cells in the sub-ventricular zone (SVZ, step 1). Upon their last division, neurons often transiently display a multipolar morphology (step 2) before switching to a unipolar morphology where the leading process (LP) is attached to a radial glial process and initiates radial translocation (step 3). During radial translocation through the IZ, the trailing process (TP) elongates rapidly and becomes the axon while the cell body of the migrating neuron is still translocating towards the cortical plate (CP; steps 3–4). At this point, the leading process is actively engaged in neuronal translocation and will become the apical dendrite upon reaching the cortical plate where it will express dendritic specific markers such as MAP2 (D; green) whereas the trailing process elongates at very rapid rate and already expresses axon-specific markers (A; red). Upon reaching the top of the CP, pyramidal neurons detach from the radial glial process (step 5) and the leading process/apical dendrite starts elongating again since subsequently-generated neurons will bypass the first-generated neurons and accumulate in an ‘inside-out’ manner (steps 6–7). (B) This cellular mechanism is conserved in migrating neurons such as granule cells in the cerebellum: upon their terminal division in the external granular layer (EGL), young post-mitotic granule cell neurons (GCN) extend a two process (step 2) which become the trailing process and ultimately the classically-defined bifurcating axon of GCN (step 4) once the leading process emerges in the radial plane and GCN initiates radial migration towards its final position in the internal granular layer (IGL). The leading process defines the dendritic domain of GCN whereas the trailing process defines the axon of GCN. Modified from [74–76].
This is highly reminiscent of axon morphogenesis characterizing granule neurons in the developing cerebellum (Figure 1B). Cerebellar granule neuron (CGN) polarity has been particularly well characterized because it is possible to purify millions of newborn CGN for use in cellular imaging, biochemical or molecular genetic assays [22,23]. Interestingly, CGN polarity is quite hardwired as purified cells transit through each morphological stage of differentiation in vitro with an almost identical time course as seen in vivo [24]. After cycle exit, nascent CGNs extend axons and eventually transition from a unipolar to bipolar morphology by elaborating two parallel fibers (Figure 1B). After axon extension is complete the CGN cell body sprouts a specialized leading process and then migrates along Bergmann glial fibers to ultimately settle within the internal granule layer (IGL) (Figure 1B).
The PAR3/PAR6 complex and neuronal polarity
Almost two decades ago, Ken Kemphues’ group performed a very successful screen aimed at identifying genes that regulate the antero-posterior polarity of the first asymmetric cell divisions of the C. elegans zygote [25–27]. This screen identified six Par genes encoding unrelated protein families (Figure 2). A large number of studies have since demonstrated that invertebrate and vertebrates Par genes play critical roles in epithelial cell polarity during development as well as in the context of cell transformation and metastasis [28,29].
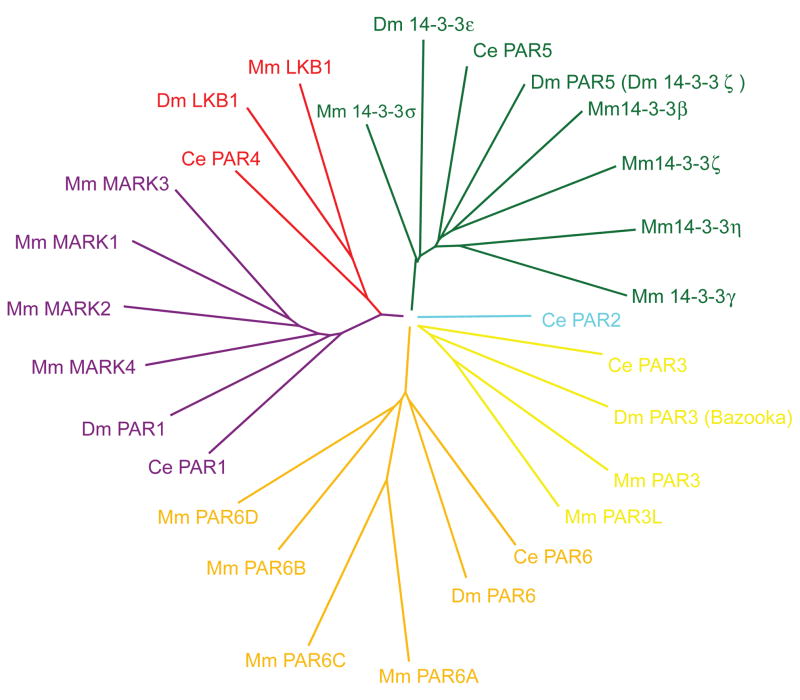
Figure 2. Phylogeny of the Par proteins family in Caenorhabditis elegans (Ce), Drosophila melanogaster (Dm) and Mus musculus (Mm).
Six Par proteins were identified in the initial screen performed to identify ‘partitioning’ defects during the first set of asymmetric cell divisions of C. elegans zygotes: Par1 encodes for a serine/threonine kinase and its four closest orthologs in mammals are called Microtubule Affinity Regulated Kinases (MARK1–4). Par2 is a Ring-finger protein but does not have any readily identified ortholog in mammals. Par3 and Par6 encodes for multi-domain scaffolding proteins which have respectively two and four orthologs in mammals. Par4 encodes for a serine/threonine kinase and its mammalian ortholog, LKB1, is unique in the mammalian genome (boxed). Finally, Par5 encodes for a 14-3-3 protein which preferentially binds to phospho-serine or phospho-threonine residues and also has multiple orthologs in mammals.
The best-studied Par genes are undoubtedly Par3 and Par6 which encode for two cytoplasmic scaffolding proteins. Par3 and Par6 can form a ternary complex containing atypical PKC (aPKC -PKCλ or PKCζ) which can recruit the small-GTPase Cdc42 and thereby regulate the dynamics of actin and microtubule cytoskeleton, epithelial cell polarity, tight junction formation, mitotic spindle orientation and cell migration [30]. Recently, Shi et al. showed that endogenous mouse (m)Par3 and mPar6 accumulate at the tip of a growing axon but not unspecified neurites, suggesting this complex is spatially and temporally activated during axon specification [12]. Ectopic expression of either PAR complex components potently inhibits hippocampal axon specification. How does the PAR complex accumulate within the nascent axon? Further analysis has recently shown that PI3-kinase activity, kinesin motors and GSK3β are required to target mPar3 to the microtubule cytoskeleton of nascent axons to facilitate axonal polarity [11]. While it is clear the Par3/Par6 complex modulates Cdc42 and Rac1 signaling which plays a role in neuronal polarization in cultured hippocampal neurons [10,31], a clear genetic loss-of-function for Par3 and Par6 in mammalian neurons is needed to determine the requirement of this protein complex for neuronal polarization in vivo. In Drosophila, neurons deficient for Bazooka (Par3 ortholog), Par6, or aPKC do not display any neuronal polarity defect [32]. Interestingly, the real-time analysis of retinal ganglion cell (RGC) polarization in vivo in zebrafish Danio rerio, demonstrates that Par3 localizes to the centrosome which localizes to the trailing part of migrating RGC becoming the dendrite [33]. Therefore, in this in vivo context, the centrosome does not localize to the part of the neuron becoming the axon as recently proposed based mostly on in vitro investigation of mammalian neurons [34].
The analysis of the role of the apical polarity complex (Par3/Par6/aPKC) is complicated by two major factors: (i) Par3/Par6/aPKC and Cdc42 are involved in controlling apico-basal polarity of dividing neural progenitors in vivo (see below and [35–37]), thus rendering any potential genetic loss-of-function phenotype rather difficult to interpret given that a defect in post-mitotic neuronal polarity may be due to a preexisting disruption in cellular architecture of neural progenitors and (ii) the fact that the mammalian genome contains at least two distinct Par3 genes and four Par6 genes. Finally, the extrinsic factors and upstream signaling mechanisms regulating the localization or the activation of the Par3/Par6 complex are poorly understood (see below for discussion of potential link between Par3/Par6 complex and the Par4-Par1 dyad; Figure 3B).
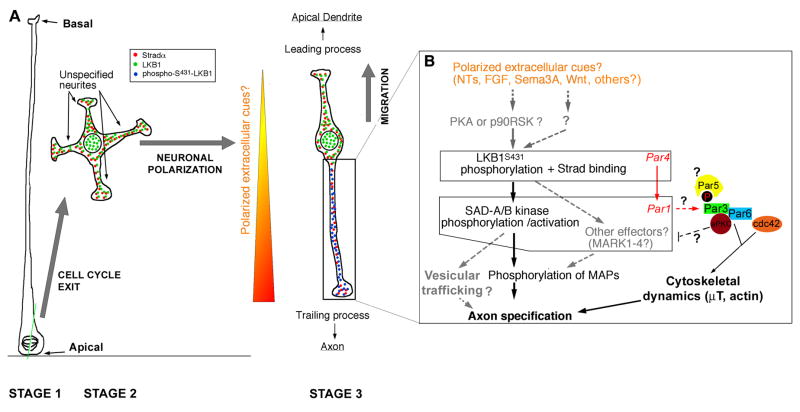
Figure 3. Molecular mechanisms underlying LKB1 function in neuronal polarization in vivo.
(A) Upon asymmetric cell division of radial glial progenitors (Stage 1), early unpolarized post-mitotic neurons show a transient phase of non-directed neurite outgrowth in the subventricular zone (Stage 2) before adopting a bipolar morphology in the intermediate zone where they engage radial migration with a leading process directed towards the pial surface and a trailing process directed towards the ventricle. (B) Based on recent reports [21,55], we propose that in vivo, the trailing process is specified to become the axon in response to putative extracellular cues that preferentially induce phosphorylation of LKB1 on Serine431. This event might be mediated in part by cues providing chemotactic attraction of radially migrating neurons towards the cortical plate such as Sema3A [70] or any other extracellular cues neurotrophins (NTs) such as BDNF/NT4/NT3 [55], Wnt [66,67], FGFs or other cues that can activate cAMP-dependent protein kinase (PKA) or p90 RSK (RSK1–3). One cannot exclude the possibility of another uncharacterized serine/threonine protein kinase can phosphorylate Serine 431 in vivo and play a role in neuronal polarization. Once LKB1 is activated by binding to its necessary co-activator Strad (αor β) and S431-phosphorylation (which occurs only in the neurite becoming the axon), LKB1 phosphorylates SAD-A/B kinases (and likely Microtubule Affinity-Regulated Kinases, MARK1–4) which are required for axon specification in part by phosphorylating microtubule-associated proteins such as Tau. Based on the function of SAD-kinases in presynaptic vesicular clustering in C. elegans [77], we can hypothesize that SAD-A/B kinases might also specify axon identity by directing vesicular trafficking in the neurite becoming the axon. Based on evidence obtained in D. melanogaster, Par1 can phosphorylate Par3 on two serine residues that constitute binding sites for the 14-3-3 protein Par5, an event that controls its localization during D. melanogaster oocyte polarity. At present this is the only potential link between the Par3/Par6/aPKC complex and Par4/Par1 dyad during cell polarization. See text for details. Modified from [21].
Role of Par6 and microtubule organizing center (MTOC) in neuronal polarization
The seminal studies of Spiegleman et al. as well as Lefcort and Bentley suggested that the position of the microtubule organization center (MTOC) correlates with neurite initiation site and could provide a scaffold underlying neuronal polarity [38,39]. In CGN dissociated and plated in vitro, the MTOC is initially located at the pole where the first parallel fiber is initiated [40], it then rotates to the opposite pole when the second fiber is formed [41]. Moreover, the MTOC is specifically directed towards the leading process of migrating neurons, and moves in the direction of migration before somal translocation [42–45•].
Is there any physiological significance to MTOC repositioning during CGN polarization? Low concentrations of actin depolymerizing drugs, which fail to block initial parallel fiber formation, inhibit MTOC repositioning and block CGNs in a unipolar morphology [41]. Overexpression or silencing of MTOC-associated Par6, which disrupts the microtubule cytoskeleton and MTOC positioning, blocks parallel fiber formation and somal translocation during radial migration [45•]. Recently, Mishra and coworkers demonstrated that GAP43 is a component of the MTOC in CGN. Genetic ablation of GAP43 leads to displacement of Par6 from the MTOC, MTOC positioning defects, extension of supernumary Tau positive parallel fibers and retarded radial migration [46]. The GAP43 phenotype suggests that the MTOC may be dispensable for polarity initiation in CGNs but may be needed to stabilize the axis of polarity in nascent axons or dendrites. While the MTOC is necessary and sufficient to drive some forms of cell polarity [47], further work is needed to determine if the MTOC alone is sufficient to initiate neuronal polarity as has been proposed by de Anda and coworkers [34]. This is an especially controversial topic since a large amount of work has demonstrated that both the centrosome and the golgi apparatus are located towards the leading process in radially migrating cortical neurons which becomes the apical dendrite in post-migratory cortical pyramidal neurons as demonstrated by reconstruction of serial electron microscopic sections [48] and more recently using real-time imaging [42–44]. However, studies performed using in vitro dissociated hippocampal neurons suggested that the centrosome and the Golgi apparatus are oriented towards the neurite becoming the axon in vitro [34] which corresponds to the trailing process in vivo [19–21,49]. Future investigations should resolve this discrepancy by examining the position and function of the centrosome and Golgi apparatus in axon-dendrite polarization using real-time imaging of migrating neurons in vivo.
LKB1 and SAD kinases: a conserved Par4/Par1 kinase pathway specifying cortical neuron polarity in vivo
While several signaling pathways have been explored for their potential function in neuronal polarity using in vitro approaches, few have been explored in an in vivo context. Recently, two studies have demonstrated the importance of the serine/threonine kinase LKB1 recently described as a regulator of neuronal polarity in the developing neocortex. LKB1 is a tumor suppressor and the mammalian ortholog of the conserved polarity gene Par4 [50]. LKB1 activation (by inducing expression of its necessary co-activator Stradα) in a colonic epithelial cell line is sufficient to induce a remarkably complete level of epithelial polarity within 24hrs [51••]. This remarkable but still mysterious result suggests that LKB1 possesses a unique bioactivity that is sufficient to induce a complete program of epithelial cell polarization in the absence of any cell-cell contact.
Recent evidence demonstrates that selective elimination of LKB1 expression in dorsal telencephalic progenitors leads to the selective loss of axon initiation in cortical neurons in vivo [21••]. Conversely, over-activation of LKB1 by co-expression with its necessary co-activator Stradα is sufficient to induce the formation of multiple axons in cortical neurons. Interestingly, LKB1 relies on a rather unconventional, allosteric mechanism of kinase activation through binding of the pseudo-kinase Stradα which also plays a critical role in exporting LKB1 out of the nucleus into the cytoplasm [52–54]. This activation step is necessary, but not sufficient, for LKB1 to execute its role in axon specification, as LKB1 must be also be phosphorylated on Serine 431 near the C-terminus of the protein [21••,55••], a site previously shown to be required for LKB1 function in oocyte polarity in Drosophila [56]. It is likely that this complex regulation scheme exists to restrict spatially and temporally the axogenic pool of LKB1 activity. In fact, whereas LKB1 shows uniform cytoplasmic distribution in unpolarized neurons, LKB1 phosphorylated on S431 is specifically enriched in a single neurite becoming the axon [21••,55••]. Shelly et al. went on to demonstrate that this S431 phosphorylation can be induced in a single immature neurite by local application of the extracellular cue BDNF in a Protein Kinase A (PKA)-dependent signaling cascade [55••] (Figure 3). These results raise several important questions: is BDNF a relevant cue in vivo to induce S431-LKB1-dependent axon specification or are other extracellular cues participating to this critical event? Biochemical studies have shown that either PKA or p90RSK (RSK1–3) can phosphorylate S431 in LKB1 [57], but which of these (or other) kinases phosphorylate S431-LKB1 in vivo during axon specification? What is the molecular mechanism(s) underlying the function of S431-LKB1? Are there phospho-specific interactors of S431-LKB1 underlying its function in axon specification? Is there a negative feed-back mechanism regulating the absence of S431-phosphorylation in neurites that become dendrites such as a phosphatase and/or is phospho-S431 protected from de-phosphorylation in the neurite becoming the axon by a 14-3-3/Par5 protein-binding mechanism? Future investigations will undoubtedly answer some of these important questions and provide more insights into the molecular mechanisms underlying axon specification during neuronal polarization.
What are the downstream effectors of LKB1 in neuronal polarization? LKB1 is considered a ‘master’ kinase because it can phosphorylate and activate at least 13 other kinases defining the ‘AMPK-like branch’ of the mammalian kinome [58,59]. Interestingly, recent evidence demonstrated that two of these kinases called SAD-A and SAD-B (also known as Brain Specific Kinases or BRSK1/2) are required in vivo for axon specification in cortical neurons [60•]. Barnes et al. provided biochemical and functional evidence demonstrating that LKB1 is the main upstream activator of SAD-A/B kinases in cortical neurons [21••]. Abnormal axon and dendrite formation have also been observed following manipulation of another LKB1 effector, MARK2, a member of the Microtubule-Affinity Regulated Kinase (MARK) sub-family of protein kinases which are the closest orthologs of C. elegans Par1 [9,61,62]. However, these results were obtained using primarily in vitro dissociated hippocampal cultures, and in vivo confirmation of the requirement of MARKs for neuronal polarization awaits further analysis using genetic loss-of-function. In fact, a MARK2 knockout mouse was produced and has no obvious axon specification phenotype [63] which might be due, at least in part, to genetic redundancy with the other three MARK kinases.
A limited number of studies have explored the link between the Par3/Par6/aPKC complex and the Par4-Par1 dyad. One study using the Drosophila oocyte polarization as a model system demonstrated that Par1 can phosphorylate Bazooka/Par3 on two distinct serine residues that constitutes binding site for the 14-3-3 protein Par5 [64]. This study reveals that this event inhibits the formation of the Bazooka/PAR-6/aPKC complex by blocking Bazooka oligomerization and binding to aPKC. In epithelial cells, this complex localizes apically and defines the apical membrane, whereas Bazooka lacking PAR-1 phosphorylation/14-3-3 binding sites forms ectopic lateral complexes [64]. Future investigation will probably test if LKB1-mediated activation of SAD-kinases (i) leads to Par3 phosphorylation and (ii) if this event controls the localization or formation of the Par3/Par6/aPKC complex and (iii) if this plays a role in axon specification.
Extracellular cues specifying neuronal polarity
Is there any evidence for the role of extracellular cues participating to the neuronal polarization in vivo? Selectively labeling of HSN motor neurons in C. elegans allows the observation of the development and extension of a single ventral axon in vivo [65••]. In this study, Cori Bargmann’s group show that in addition to axon guidance, UNC-6/netrin is required for the initial break in neuronal symmetry that precedes the future asymmetric growth of the axon. Interestingly, UNC-6/Netrin signaling causes MIG10 (a member of a family that includes RIAM and lamellipodin) to localize to the leading edge of the presumptive axon. MIG10 is a pleckstrin homology (PH)-domain containing protein and this study shows that mutations in age-1/PI3-Kinase and daf-18/PTEN, which regulate intracellular PIP2 levels, also alter UNC-6/Netrin-induced MIG10 localization. Another set of secreted cues, Wnt, has also been shown to play a similar role in controlling the asymmetry of axon outgrowth during neuronal polarization in C. elegans [66•,67•]. Taken together, these results suggest that in C.elegans both Netrin and Wnt play a key role in polarizing axon growth in vivo.
Is there any evidence for the role of extracellular cues in polarizing the asymmetric emergence of the axon in vertebrate neurons? Class 3 secreted semaphorin, Sema3A, has been shown to play a role in specifying the orientation of axon apical dendrite growth in cortical neurons. Using a slice overlay assay as well as analysis of Sema3A-deficient mice, Polleux et al. demonstrated that a gradient of Sema3A within the cortical wall is repulsing axons ventrally (away from the cortical plate) and attracting the apical dendrite dorsally (towards the cortical plate) [68•,69]. Recently, it has been shown that Sema3A and its main receptor Neuropilin1 control the orientation of leading process outgrowth in radially migrating cortical neurons in the intermediate zone [70]. The leading process becomes the apical dendrite in cortical pyramidal neurons, therefore providing a model for how the apical dendrite of pyramidal neurons becomes oriented towards the pial surface as a chemoattractive response to Sema3A. Future investigations will test whether the LKB1/SAD kinases pathway lies downstream of Sema3A or any other extracellular cues directing leading/trailing process outgrowth during neuronal polarization which in turns underlies axon/dendrite polarity in vivo (Figure 3).
Is there a link between the apico-basal polarity of dividing neural progenitors and axon-dendrite neuronal polarity?
During cortical development as well as in other parts of the developing central nervous system of mammals, neurons are generated by a specialized set of progenitors called radial glial cells which are characterized by a strong apico-basal polarity and a cell cycle-related interkinetic nuclear movement [71]. The basal attachment of these progenitors is contacting the basal membrane at the pial surface whereas their apical membrane is tightly sealed by adherens junctions at the ventricular border. During neurogenesis, radial glial progenitors can divide asymmetrically to generate either (1) another radial glial cell and a post-mitotic neuron engaging migration or (2) to give rise to a radial glial progenitor and a transient amplifying progenitor that can divide in an abventricular position in the subventricular zone (Figure 1A). Radial glial progenitors are highly polarized cells showing asymmetrical distribution of specific organelles such as the centrosome (always found apically) or specific transmembrane or cytoplasmic proteins [71,72]. What is the link (if any) between the apico-basal polarity of radial glial progenitors, the polarity of migrating neurons (trailing vs leading process) and axon-dendrite neuronal polarity.
As mentioned above, the Par3/Par6 complex has been shown to control neuronal polarity. This complex contains another critical component of polarity signaling atypical protein kinase C (aPKC). Both mammalian orthologs of aPKC (λ and ζ) are expressed in the developing cortex and interestingly, loss of Cdc42 (another component of PAR3/PAR6 complex) expression leads to a loss of aPKC localization in neural progenitors [35,36]. Recent analysis of conditional deleted PKCλ in the mouse brain indicates at least partial redundancy with PKCζ, since, unlike loss of Cdc42, loss of PKCλ does not appear to have a profound effect on neurogenesis during development [73]. Severe perturbation of the apical process of radial glial cells is observed in conditional PKCλ-null mice similar to that seen in cdc42-null cortical progenitors [35,36]. This is likely due a loss of adherens junctions integrity in cortical progenitors that leads to a disorganized ventricular zone, but surprisingly this does not have any significant effect on neuronal differentiation or migration, suggesting that some aspects of progenitor polarity may not impact their overall function [73]. The consequence of genetic loss-of-function of Par3/Par6, Cdc42, aPKC on neuronal polarity remains to be fully assessed, but based on the reported effect of this conserved polarity complex on neural progenitors polarity, one will have to devise a paradigm differentially addressing their effects on progenitor polarity versus post-mitotic/migrating neuron polarity. Future investigations will probably test this important question in vivo using combinations of high-resolution real-time imaging, fluorescent reporters and genetic approaches in vivo.
Conclusion
Over the past two decades, important progress has been made in identifying candidate molecules regulating the establishment of axon-dendrite polarity during mammalian development using primarily in vitro approaches. In multiple systems, it is now apparent that this aspect of neuronal polarity is tightly linked to the molecular mechanisms polarizing the leading and the trailing processes of neurons as they engage migration. An important challenge facing this field is to identify how extracellular cues regulate the distribution and/or activation of intrinsic factors underlying the brake of ‘neuronal symmetry’ which leads to the polarized emergence of axon and dendrites in vivo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- 3.Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- 4.Yu W, Cook C, Sauter C, Kuriyama R, Kaplan PL, Baas PW. Depletion of a microtubule-associated motor protein induces the loss of dendritic identity. J Neurosci. 2000;20:5782–5791. doi: 10.1523/JNEUROSCI.20-15-05782.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Da Silva JS, Hasegawa T, Miyagi T, Dotti CG, Abad-Rodriguez J. Asymmetric membrane ganglioside sialidase activity specifies axonal fate. Nat Neurosci. 2005;8:606–615. doi: 10.1038/nn1442. [DOI] [PubMed] [Google Scholar]
- 6.Inagaki N, Chihara K, Arimura N, Menager C, Kawano Y, Matsuo N, Nishimura T, Amano M, Kaibuchi K. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4:781–782. doi: 10.1038/90476. [DOI] [PubMed] [Google Scholar]
- 7.Menager C, Arimura N, Fukata Y, Kaibuchi K. PIP3 is involved in neuronal polarization and axon formation. J Neurochem. 2004;89:109–118. doi: 10.1046/j.1471-4159.2004.02302.x. [DOI] [PubMed] [Google Scholar]
- 8.Jiang H, Guo W, Liang X, Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 9.Chen YM, Wang QJ, Hu HS, Yu PC, Zhu J, Drewes G, Piwnica-Worms H, Luo ZG. Microtubule affinity-regulating kinase 2 functions downstream of the PAR-3/PAR-6/atypical PKC complex in regulating hippocampal neuronal polarity. Proc Natl Acad Sci U S A. 2006;103:8534–8539. doi: 10.1073/pnas.0509955103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwamborn JC, Puschel AW. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat Neurosci. 2004;7:923–929. doi: 10.1038/nn1295. [DOI] [PubMed] [Google Scholar]
- 11.Shi SH, Cheng T, Jan LY, Jan YN. APC and GSK-3beta are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr Biol. 2004;14:2025–2032. doi: 10.1016/j.cub.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112:63–75. doi: 10.1016/s0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimura T, Arimura N, Kawano Y, Kawabata S, Wang S, Kaibuchi K. Ras regulates neuronal polarity via the PI3-kinase/Akt/GSK-3beta/CRMP-2 pathway. Biochem Biophys Res Commun. 2006;340:62–68. doi: 10.1016/j.bbrc.2005.11.147. [DOI] [PubMed] [Google Scholar]
- 15 •.Watabe-Uchida M, John KA, Janas JA, Newey SE, Van Aelst L. The Rac activator DOCK7 regulates neuronal polarity through local phosphorylation of stathmin/Op18. Neuron. 2006;51:727–739. doi: 10.1016/j.neuron.2006.07.020. This study provides evidence that DOCK7 and the small GTPase Rac signalling regulate axon specification at least in part by regulating phosphorylation and inactivation of the microtubule destabilizating protein Stathmin/Op18. This and other studies [4,16] points to the critical role of regulating microtubules dynamics during axon specification. [DOI] [PubMed] [Google Scholar]
- 16.Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz ML, Rakic P, Goldman-Rakic PS. Early phenotype expression of cortical neurons: evidence that a subclass of migrating neurons have callosal axons. Proc Natl Acad Sci U S A. 1991;88:1354–1358. doi: 10.1073/pnas.88.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatanaka Y, Murakami F. In vitro analysis of the origin, migratory behavior, and maturation of cortical pyramidal cells. J Comp Neurol. 2002;454:1–14. doi: 10.1002/cne.10421. [DOI] [PubMed] [Google Scholar]
- 20.Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 21 ••.Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. Together with [55••], this study identifies the serine/threonine kinase LKB1 as a critical regulator of neuronal polarization in vivo. Conditional deletion of LKB1 expression results in a specific inhibition of axon formation in cortical neurons as they engage migration. The authors also show that LKB1 mediate its effect on axon specification at least in part by phosphorylating and activating SAD-A/B kinases, previously shown to be required in vivo for axon specification (see [60 •]). [DOI] [PubMed] [Google Scholar]
- 22.Edmondson JC, Hatten ME. Glial-guided granule neuron migration in vitro: a high-resolution time-lapse video microscopic study. J Neurosci. 1987;7:1928–1934. doi: 10.1523/JNEUROSCI.07-06-01928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatten ME. Neuronal regulation of astroglial morphology and proliferation in vitro. J Cell Biol. 1985;100:384–396. doi: 10.1083/jcb.100.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell SK, Rivas RJ, Rodriguez-Boulan E, Hatten ME. Development of polarity in cerebellar granule neurons. J Neurobiol. 1997;32:223–236. doi: 10.1002/(sici)1097-4695(199702)32:2<223::aid-neu7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 25.Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52:311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- 26.Rose LS, Kemphues KJ. Early patterning of the C. elegans embryo. Annu Rev Genet. 1998;32:521–545. doi: 10.1146/annurev.genet.32.1.521. [DOI] [PubMed] [Google Scholar]
- 27.Watts JL, Morton DG, Bestman J, Kemphues KJ. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development. 2000;127:1467–1475. doi: 10.1242/dev.127.7.1467. [DOI] [PubMed] [Google Scholar]
- 28.Wodarz A, Nathke I. Cell polarity in development and cancer. Nat Cell Biol. 2007;9:1016–1024. doi: 10.1038/ncb433. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munro EM. PAR proteins and the cytoskeleton: a marriage of equals. Curr Opin Cell Biol. 2006;18:86–94. doi: 10.1016/j.ceb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, Hoshino M, Kaibuchi K. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol. 2005;7:270–277. doi: 10.1038/ncb1227. [DOI] [PubMed] [Google Scholar]
- 32.Rolls MM, Doe CQ. Baz, Par-6 and aPKC are not required for axon or dendrite specification in Drosophila. Nat Neurosci. 2004;7:1293–1295. doi: 10.1038/nn1346. [DOI] [PubMed] [Google Scholar]
- 33.Zolessi FR, Poggi L, Wilkinson CJ, Chien CB, Harris WA. Polarization and orientation of retinal ganglion cells in vivo. Neural Develop. 2006;1:2. doi: 10.1186/1749-8104-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Anda FC, Pollarolo G, Da Silva JS, Camoletto PG, Feiguin F, Dotti CG. Centrosome localization determines neuronal polarity. Nature. 2005;436:704–708. doi: 10.1038/nature03811. [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Liao G, Yang L, Campbell K, Nakafuku M, Kuan CY, Zheng Y. Cdc42 deficiency causes Sonic hedgehog-independent holoprosencephaly. Proc Natl Acad Sci U S A. 2006;103:16520–16525. doi: 10.1073/pnas.0603533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cappello S, Attardo A, Wu X, Iwasato T, Itohara S, Wilsch-Brauninger M, Eilken HM, Rieger MA, Schroeder TT, Huttner WB, et al. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci. 2006;9:1099–1107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- 37.Costa MR, Wen G, Lepier A, Schroeder T, Gotz M. Par-complex proteins promote proliferative progenitor divisions in the developing mouse cerebral cortex. Development. 2008;135:11–22. doi: 10.1242/dev.009951. [DOI] [PubMed] [Google Scholar]
- 38.Lefcort F, Bentley D. Organization of cytoskeletal elements and organelles preceding growth cone emergence from an identified neuron in situ. J Cell Biol. 1989;108:1737–1749. doi: 10.1083/jcb.108.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiegelman BM, Lopata MA, Kirschner MW. Aggregation of microtubule initiation sites preceding neurite outgrowth in mouse neuroblastoma cells. Cell. 1979;16:253–263. doi: 10.1016/0092-8674(79)90003-5. [DOI] [PubMed] [Google Scholar]
- 40.Zmuda JF, Rivas RJ. The Golgi apparatus and the centrosome are localized to the sites of newly emerging axons in cerebellar granule neurons in vitro. Cell Motil Cytoskeleton. 1998;41:18–38. doi: 10.1002/(SICI)1097-0169(1998)41:1<18::AID-CM2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 41.Zmuda JF, Rivas RJ. Actin filament disruption blocks cerebellar granule neurons at the unipolar stage of differentiation in vitro. J Neurobiol. 2000;43:313–328. doi: 10.1002/1097-4695(20000615)43:4<313::aid-neu1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 42.Higginbotham H, Tanaka T, Brinkman BC, Gleeson JG. GSK3beta and PKCzeta function in centrosome localization and process stabilization during Slit-mediated neuronal repolarization. Mol Cell Neurosci. 2006;32:118–132. doi: 10.1016/j.mcn.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka T, Serneo FF, Higgins C, Gambello MJ, Wynshaw-Boris A, Gleeson JG. Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J Cell Biol. 2004;165:709–721. doi: 10.1083/jcb.200309025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai JW, Bremner KH, Vallee RB. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat Neurosci. 2007;10:970–979. doi: 10.1038/nn1934. [DOI] [PubMed] [Google Scholar]
- 45 •.Solecki DJ, Model L, Gaetz J, Kapoor TM, Hatten ME. Par6alpha signaling controls glial-guided neuronal migration. Nat Neurosci. 2004;7:1195–1203. doi: 10.1038/nn1332. This study provides the first evidence that Par6alpha is localized to the MTOC where it is critical for proper polarized migration of granule cell neurons. [DOI] [PubMed] [Google Scholar]
- 46.Mishra R, Gupta SK, Meiri KF, Fong M, Thostrup P, Juncker D, Mani S. GAP-43 is key to mitotic spindle control and centrosome-based polarization in neurons. Cell Cycle. 2007;7 doi: 10.4161/cc.7.3.5235. [DOI] [PubMed] [Google Scholar]
- 47.Cowan CR, Hyman AA. Centrosomes direct cell polarity independently of microtubule assembly in C. elegans embryos. Nature. 2004;431:92–96. doi: 10.1038/nature02825. [DOI] [PubMed] [Google Scholar]
- 48.Shoukimas GM, Hinds JW. The development of the cerebral cortex in the embryonic mouse: an electron microscopic serial section analysis. J Comp Neurol. 1978;179:795–830. doi: 10.1002/cne.901790407. [DOI] [PubMed] [Google Scholar]
- 49.Hatanaka Y, Hisanaga S, Heizmann CW, Murakami F. Distinct migratory behavior of early-and late-born neurons derived from the cortical ventricular zone. J Comp Neurol. 2004;479:1–14. doi: 10.1002/cne.20256. [DOI] [PubMed] [Google Scholar]
- 50.Alessi DR, Sakamoto K, Bayascas JR. LKB1-Dependent Signaling Pathways. Annu Rev Biochem. 2006 doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 51 ••.Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. A remarkable study demonstrating for the first time that activation of a kinase (LKB1) is sufficient to initiate a complete program of polarization in a colonic epithelial cell line in the absence of cell-cell contact. [DOI] [PubMed] [Google Scholar]
- 52.Boudeau J, Scott JW, Resta N, Deak M, Kieloch A, Komander D, Hardie DG, Prescott AR, van Aalten DM, Alessi DR. Analysis of the LKB1-STRAD-MO25 complex. J Cell Sci. 2004;117:6365–6375. doi: 10.1242/jcs.01571. [DOI] [PubMed] [Google Scholar]
- 53.Boudeau J, Baas AF, Deak M, Morrice NA, Kieloch A, Schutkowski M, Prescott AR, Clevers HC, Alessi DR. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. Embo J. 2003;22:5102–5114. doi: 10.1093/emboj/cdg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dorfman J, Macara IG. STRAD{alpha} Regulates LKB1 Localization by Blocking Access To Importin-{alpha}, and by Association With Crm1 and Exportin-7. Mol Biol Cell. 2008 doi: 10.1091/mbc.E07-05-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55 ••.Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–577. doi: 10.1016/j.cell.2007.04.012. Together with [21••], this study identifies LKB1 as a critical regulator of axon specification. Shelly et al. show that in vitro, the ability of localized application of an extracellular cue such as BDNF to specify one neurite to become the axon requires phosphorylation of LKB1 on Serine 431 by cAMP-dependent protein kinase (PKA). [DOI] [PubMed] [Google Scholar]
- 56.Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature. 2003;421:379–384. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- 57.Sapkota GP, Kieloch A, Lizcano JM, Lain S, Arthur JS, Williams MR, Morrice N, Deak M, Alessi DR. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell vrowth. J Biol Chem. 2001;276:19469–19482. doi: 10.1074/jbc.M009953200. [DOI] [PubMed] [Google Scholar]
- 58.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. Embo J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60 •.Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science. 2005;307:929–932. doi: 10.1126/science.1107403. This article identifies SAD-kinases as critical regulators of neuronal polarization of cortical neurons in vivo. The authors produced double knockout mice for SAD-A and SAD-B kinases and show that cortical neurons are unable to specify a single neurite to become an axon in vivo. [DOI] [PubMed] [Google Scholar]
- 61.Biernat J, Wu YZ, Timm T, Zheng-Fischhofer Q, Mandelkow E, Meijer L, Mandelkow EM. Protein kinase MARK/PAR-1 is required for neurite outgrowth and establishment of neuronal polarity. Mol Biol Cell. 2002;13:4013–4028. doi: 10.1091/mbc.02-03-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Terabayashi T, Itoh TJ, Yamaguchi H, Yoshimura Y, Funato Y, Ohno S, Miki H. Polarity-regulating kinase partitioning-defective 1/microtubule affinity-regulating kinase 2 negatively regulates development of dendrites on hippocampal neurons. J Neurosci. 2007;27:13098–13107. doi: 10.1523/JNEUROSCI.3986-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hurov JB, Stappenbeck TS, Zmasek CM, White LS, Ranganath SH, Russell JH, Chan AC, Murphy KM, Piwnica-Worms H. Immune system dysfunction and autoimmune disease in mice lacking Emk (Par-1) protein kinase. Mol Cell Biol. 2001;21:3206–3219. doi: 10.1128/MCB.21.9.3206-3219.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/s0092-8674(03)00938-3. [DOI] [PubMed] [Google Scholar]
- 65 ••.Adler CE, Fetter RD, Bargmann CI. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat Neurosci. 2006;9:511–518. doi: 10.1038/nn1666. A remarkable analysis of axon initiation at single cell resolution in C. elegans demonstrates that Netrin released by underlying muscle cells regulates the ventral site of axon initiation in HSN neuron. Together with [66•, 67• and 68•], an emerging model suggests that in invertebrates and maybe vertebrates neurons, extracellular cues such as Netrin, Wnt or semaphorins direct the polarity of axon initiation using a chemotactic-like mechanism in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66 •.Prasad BC, Clark SG. Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development. 2006;133:1757–1766. doi: 10.1242/dev.02357. See [65••]. [DOI] [PubMed] [Google Scholar]
- 67 •.Hilliard MA, Bargmann CI. Wnt signals and frizzled activity orient anterior-posterior axon outgrowth in C. elegans. Dev Cell. 2006;10:379–390. doi: 10.1016/j.devcel.2006.01.013. See [65••]. [DOI] [PubMed] [Google Scholar]
- 68 •.Polleux F, Giger RJ, Ginty DD, Kolodkin AL, Ghosh A. Patterning of cortical efferent projections by semaphorin-neuropilin interactions. Science. 1998;282:1904–1906. doi: 10.1126/science.282.5395.1904. See [65••]. [DOI] [PubMed] [Google Scholar]
- 69.Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- 70.Chen G, Sima J, Jin M, Wang KY, Xue XJ, Zheng W, Ding YQ, Yuan XB. Semaphorin-3A guides radial migration of cortical neurons during development. Nat Neurosci. 2008;11:36–44. doi: 10.1038/nn2018. [DOI] [PubMed] [Google Scholar]
- 71.Guerrier S, Polleux F. The ups and downs of neural progenitors: Cep120 and TACCs control interkinetic nuclear migration. Neuron. 2007;56:1–3. doi: 10.1016/j.neuron.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 72.Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 73.Imai F, Hirai S, Akimoto K, Koyama H, Miyata T, Ogawa M, Noguchi S, Sasaoka T, Noda T, Ohno S. Inactivation of aPKCλ results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133:1735–1744. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- 74.Gao WQ, Hatten ME. Neuronal differentiation rescued by implantation of Weaver granule cell precursors into wild-type cerebellar cortex. Science. 1993;260:367–369. doi: 10.1126/science.8469990. [DOI] [PubMed] [Google Scholar]
- 75.Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol. 1971;141:283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- 76.Komuro H, Yacubova E, Rakic P. Mode and tempo of tangential cell migration in the cerebellar external granular layer. J Neurosci. 2001;21:527–540. doi: 10.1523/JNEUROSCI.21-02-00527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crump JG, Zhen M, Jin Y, Bargmann CI. The SAD-1 kinase regulates presynaptic vesicle clustering and axon termination. Neuron. 2001;29:115–129. doi: 10.1016/s0896-6273(01)00184-2. [DOI] [PubMed] [Google Scholar]