Abstract
The cartilage is composed of chondrocytes embedded in a matrix of collagen fibrils interspersed within a network of proteoglycans and is constantly exposed to biomechanical forces during normal joint movement. Characterization of the surface morphology, cytoskeletal structure, adherance and elastic properties of these mechanotransductive cells are crucial in understanding the effects of mechanical forces around a cell and how a cell responds to changes in its physical environment. In this work, we employed the atomic force microscope (AFM) to image cultured chondrocytes before and after subjecting them to mechanical forces in the presence or absence of interleukin-1β to mimic inflammatory conditions. Nanoscale imaging and quantitative measurements from AFM data revealed that there are distinct changes in cell surface topology and cytoskeleton arrangement in the cells following treatment with mechanical forces, IL-1β or both. Our findings for the first time demonstrate that cultured chondrocytes are amenable to high-resolution AFM imaging and dynamic tensile forces may help overcome the effect of inflammatory factors on chondrocyte response.
Keywords: chondrocytes, mechanical strain, AFM, cytoskeleton
INTRODUCTION
Arthritic diseases are chronic inflammatory diseases of the joints associated with significant cartilage erosion resulting in compromised joint function. Increased production of cytokines including interleukin-1β (IL-1β) by the synoviocytes and chondrocytes provide evidence for their involvement in the pathogenesis of arthritic diseases, Weissmann (2006). These cytokines upregulate transcription of proinflammatory genes to initiate cartilage destruction and amplify immune responses, Moreland (2004), Ji H (2002), Kay (2004), LeGrand (2001). Although, antiinflammatory drugs are the choice treatment, the therapeutic potential of joint mobilization in restoring joint function is increasingly being recognized Renner (2006), Sharma (2007), Strombeck (2007), Wolf (2007). It is understood that the signals associated with joint mobilization are essential for cartilage homeostasis, as well as its repair and smooth functioning of the joints, Ferretti et al. (2006).
To understand how mechanical stresses affect articular cartilage functions, a number of studies have been performed both in vivo as well as on cartilage explants and chondrocytes monolayer culture, Mobasheri et. al.(2002), Guilak et. al. (2006). Load induced deformation of cartilage matrix can cause alterations in hydrostatic pressure, ionic and osmotic composition, interstitial fluid and streaming potentials. Chondrocytes embedded in the cartilage matrix constantly experience these stimuli, Ateshian (2007). These mechanosensitive cells then respond by bringing about changes in the gene expression, protein synthesis, matrix composition and ultimately biomechanical competence of the tissue, Sharma et. al.(2007), Garcia et al. (1999), Goldmann et al. (2002). At high or traumatic magnitudes biomechanical signals trigger expression of proinflammatory genes in chondrocytes, and at low physiological magnitudes these signals are potent inhibitors of IL-1β dependent proinflammatory gene transcription, Hsieh et al. (2005), Ingber (1997), Agarwal et al. (2004). Furthermore, at low magnitudes these signals induce proteoglycan and collagen type II synthesis essential for cartilage homeostasis and repair, Agarwal et al. (2001), Gassner et al. (2000), Xu et al. (2000). During both processes, chondrocytes react with surrounding matrix, which results in alterations in their morphology, surface topography, gene expression and cytoskeletal organization. Cell surface proteins, receptors, and ion channels serve as the key interface between the cytoskeleton and the interaction of chondrocytes with the peri or extra-cellular matrix. Their density, distribution, and clustering crucially affect the cells response to external or internal stimuli. While biochemical assays are useful in revealing the expression level of these proteins Guilak et. al. (2006), Madhavan et. al. (2007), Salter D. (2004), ultrastuctural studies can provide unique information regarding their localization with respect to cell-membrane or cytoskeletal features. However, as yet neither the changes in superficial topography of chondrocytes, nor the arrangement of cytoskeleton in response to biomechanical forces and/or inflammatory factors has yet been investigated at high magnifications.
Atomic force microscopy (AFM) can serve as a valuable tool to elucidate the ultrastructural changes in cell-surface topography at the nanoscale level to appreciate changes in the distribution of cell-surface molecules that accompany biochemical or biomechanical signal-induced surface-changes. These changes may potentially provide novel insights into the control of cell shape and their interactions with pericellular matrix during activation of chemical and mechanical signaling pathways. Secondly, AFM can serve as a valuable tool to provide insights into remodeling of the actin cytoskeleton and resulting changes in the cell such as in its mechanical properties, membrane tension and mechanosensitivity.
In this work, to investigate the effects of inflammation and joint mobilization at the ultrastructural level, we have examined the changes in surface topography of chondrocytes in response to mechanical strain and proinflammatory environment. Using fluorescence light microscopy and AFM we investigated the changes in topological details of cultured chondrocytes following application of dynamic tensile forces and/or interleukin-1 (IL-1β). Our aims were to (1) to elucidate qualitative and qualitative changes in the surface topology of chondrocytes under above conditions, (2) to elucidate accompanying cytoskeletal remodeling, and (3) to investigate whether DTF can induce topological and/or cytoskeletal changes even in the presence of IL-1β. These investigations would provide novel insights into the physical and morphological properties of chondrocytes and how they may interact with the pericellular matrix in healthy and diseased tissue.
EXPERIMENTAL METHODS
Cell culture
Chondrocytes isolated from the superficial layers of the articular cartilage from the knees of 10–12 weeks old Sprague-Dawly rats were grown in HAM’s/F12 (Cellgro®, VA) containing 10% FBS (Hyclone®, UT) and used between 2nd and 3rd passage. Subsequently, cells were grown on collagen I coated flexible bottom plate (Flexercell International, NC) to 80% confluence and subjected to dynamic tensile forces (DTF) of 3% magnitude at 0.25 Hz for 24 hours, which is in the physiological range, Xu et. al. (2000). In addition, to examine the changes that may occur in chondrocytes due to mechanical and/or proinflammatory signals, these cells were also treated with interleukin-1 (IL-1β) (1 ng/ml) for 24 hrs or concurrently with IL-1β + DTF for 24 hrs. Unstretched cells were used as controls.
Fluorescence Microscopy
To analyze chondrocyte cytoskeleton arrangement, pie shaped slices of the flexible membranes containing cultured chondrocytes were cut, fixed with formaldehyde, and the chondrocytes were permeabilized in PBS containing 0.5%Triton X-100 for 30 min. The cells were stained with FITC-conjugated phalloidin (1:200) (Invitrogen Corporation, CA) for 20 min to visualize filaments of β-actin. Cell nuclei were stained by incubating the samples with 1 µg/ml DAPI in PBS for 1 minute, then washed 2 times with PBS to remove the triton-X 100. The images were acquired using an Axioplan 2 fluorescence microscope (Carl Zeiss, NY).
Atomic Force Microscopy (AFM)
AFM imaging was performed on glutaraldehyde fixed sections of flexible membranes using a Multimode AFM instrument (Veeco, Santa Barbara, CA) equipped with a Nanoscope IIIa controller. Sections of membrane were glued with epoxy to metal specimen discs and imaged under PBS in Tapping Mode. NP-S silicon nitride probes (Veeco, Santa Barbara, CA) with a nominal tip radius of 10 nm and spring constant of 0.32 N/m were used at the resonant frequency of ~ 9–10 KHz in fluid. Images were acquired at 512 × 512 lines per scan direction using a scan rate of 0.8 to 1.5 Hz. Images were recorded for the height and amplitude channels. The height images were flattened using the Nanoscope software. Vertical height of the cell surface features was estimated using the “Section analysis” tool of the Nanoscope software on the AFM height images. At least 3 independent specimens in each experimental condition were observed under AFM and data was collected from at least n=25 different cells for each sample. At least n=5 different regions from AFM images, encompassing a surface area of 100 to 400 µm2 on the cell-surface were analyzed for each sample to estimate the density and the size of granular structures. The statistical analyses done for particle size and particle density was weighted using one-way ANOVA, with samples considered correlated and significance at the p<0.01 level.
Analysis of β-actin expression
To examine the expression of β-actin, total RNA was extracted from the cells using the RNeasy Kit (Qiagen Inc, Valencia, CA) immediately following termination of DTF. A total of 1µg of RNA was reverse transcribed using the Superscript III Reverse Transcriptase Kit (Invitrogen). Expression of β-actin and Glyceraldehyde phosphate dehydrogenase (GAPDH, used as an internal control) was detected by end-point RT-PCR as described earlier (Deschner et al., 2006). Custom-designed (Primer Express, Applied Biosystems, CA), gene-specific primers were used to amplify the cDNA in Platinum PCR SuperMix (Invitrogen). The primers used were for β-actin (429 bp): forward: TGCTATGTTGCCCTAGACTTCG and reverse: CTTGCTGATCCACATCTGCTG; and for GAPDH (323 bp) forward 3’AGACAGCCGCATCTTCTTGT5’ and reverse 3’TACTCAGCACCAGCATCACC5’. The synthesis of β-actin by chondrocytes exposed to various treatments was assessed by Western Blot analysis using rabbit anti β-actin antibodies (1:5000 dilution; Abcam, CA) and horse radish peroxidase labeled goat anti rabbit IgG (1: 2000 dilution; Santa Cruz Biotechnology, CA) (Deschner et al 2006).
RESULTS
Dynamic tensile forces (DTF) changes the surface topography of chondrocytes
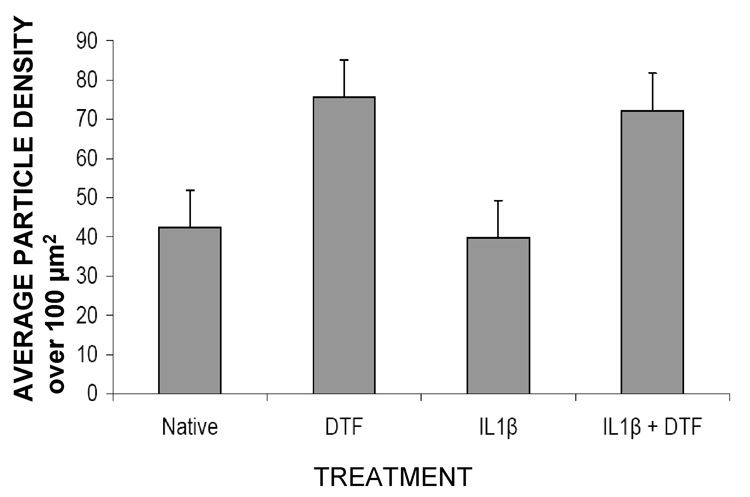
AFM imaging of cell surfaces from three independent experiments showed that native chondrocytes had a relatively smooth surface with very fine granular structures present on the cell-surface (figure 1a). In cells treated with IL1β, the cell-surface topography was similar with a few larger globular structures also present on the cell surfaces (figure 1 b). When both these samples were subjected to DTF for 24 hrs, striking changes in cell-surface topography were observed (figure 1 c, d). The stretched cells showed a marked increase in the number of granular structures on their surface, as compared to their unstretched counterparts. As shown in figure 2, while the unstretched native and IL1βtreated cells had a cell-surface particle density of 0.4 particles/µm2, the stretched cells had an average of 0.75 particles/µm2 on their surfaces showing a two-fold increase in particle density.
Figure 1.
AFM amplitude images of native chondrocytes and those subjected to IL1β treatment and/or stretch (DTF) for 24 hrs as indicated. The surface morphology of stretched cells is more granular as compared to unstretched cells. Remodeling of the cytoskeleton can also be observed for DTF treated cells as compared to their unstretched counterparts.
Figure 2.
The average number of granules per 100 µm2 surface area of the cells (as in figure 1) is quantified. Samples with DTF have an increased granular density as compared to unstretched cells with p < 0.01.
A quantitative analysis of the size distribution of these granular structures was performed by measuring the vertical height of clearly identifiable granules above the cell surface for n=50 granules (figure 3). As seen in figure 3 and figure 4, there were significant changes in the size distribution of cell-surface granules for stretched or IL1β treated cells as compared to native cells. The average granular size for native cells was 74 ± 21 nm while that for DTF or IL1β treated cells was 147 ± 50 and 104 ± 27 nm respectively. IL1β treated cells when subjected to DTF, did not differ significantly in their size distribution but had a slightly higher average size of the granular structures (125 ± 30 nm).
Figure 3.
High magnification AFM height images showing relative sizes of granular structures on the surfaces of DTF treated cells. Inset in each image shows a quantitative vertical profile along a line across the AFM image, which enables measurement of the height of granular structures. Statistical distribution of the sizes of granular structures for unstretched cells (black) vs. DTF treated (grey) is shown in for (e) native and (f) IL1-β treated cells.
Figure 4.
Average particle size for cell-surface granules ascertained from AFM images for various cell samples as indicated. Samples with DTF have a higher average particle size than their unstretched counterparts with p < 0.01.
Dynamic tensile forces (DTF) modulates cytoskeletal remodeling in chondrocytes
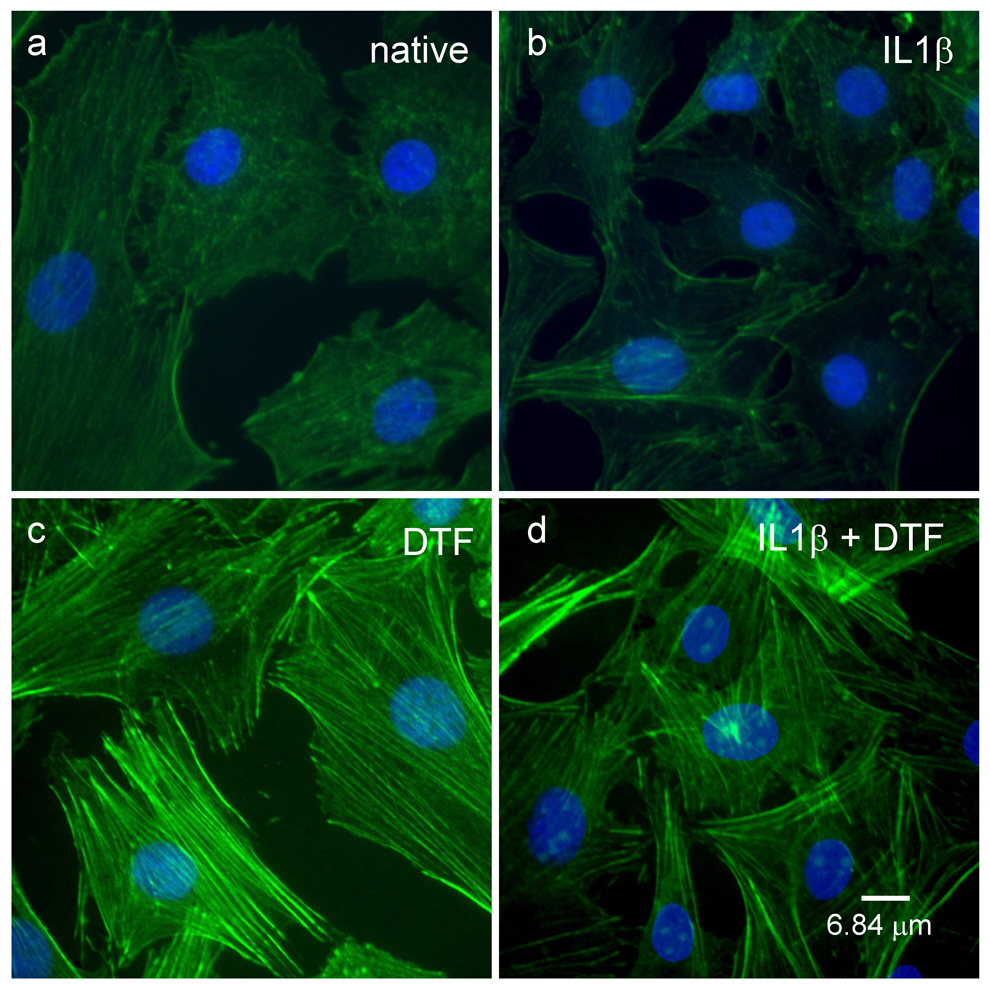
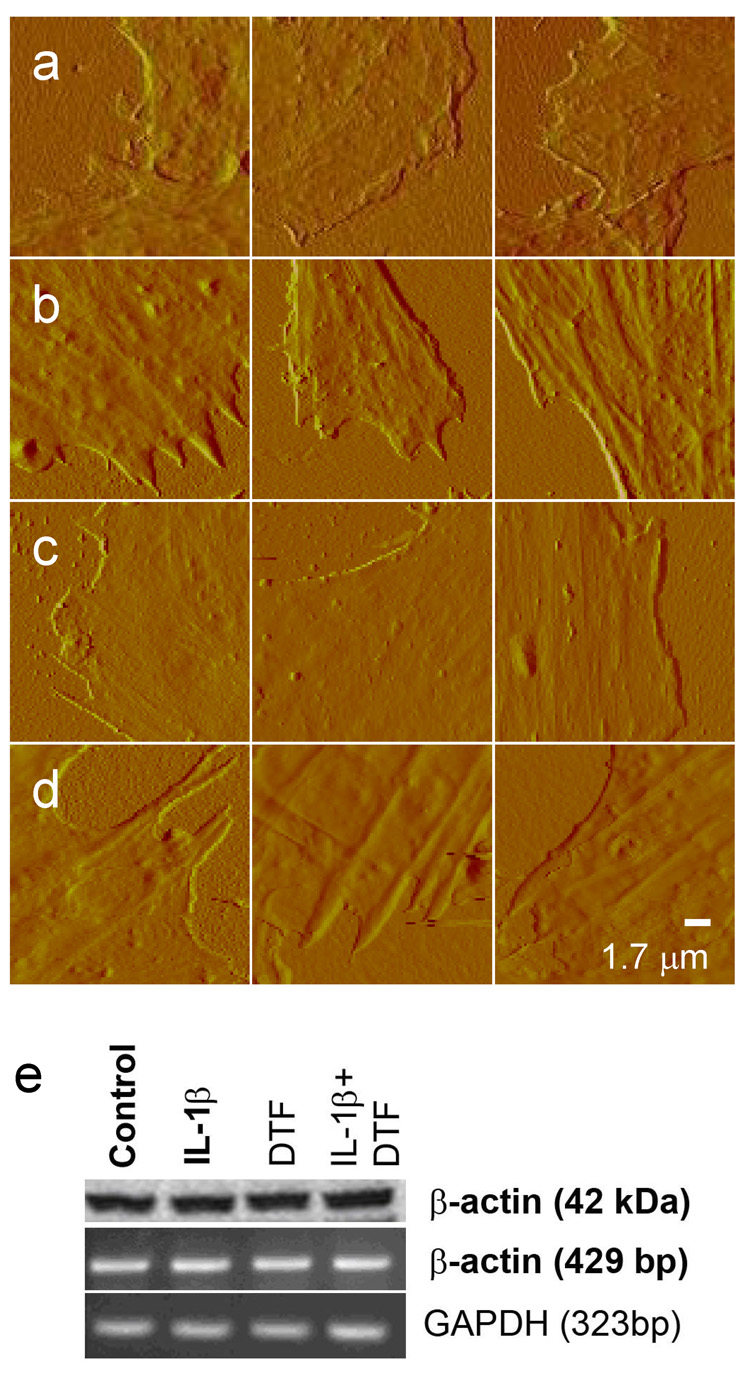
Both fluorescence microscopy and AFM images of cells showed that the cytoskeletal arrangement in chondrocytes was modified in response DTF treatment both in the absence or presence of IL1β Staining for the cytoskeletal β-actin showed that the density and distribution of actin was similar for IL1β treated or native cells (figure 5a and b). However, treatment with DTF for both these samples, led to enhanced actin polymerization with the actin fibers localized in the peripheral region of the cells (Fig 5 c–d). AFM images confirmed these observations and further revealed that native or IL1β treated cells when subjected to DTF have a well-formed cytoskeleton which extends to the peripheral regions (figure 6a–d). No changes in mRNA or protein expression levels of β-actin were observed in these experiments (figure 6e).
Figure 5.
Fluorescence microscopy images showing arrangement of FITC-stained (green) β-actin filaments in (a) native, (b) IL-1β treated, (c) DTF treated and (d) DTF and IL-1β treated chondrocytes. Nuclei were stained with DAPI, shown in blue.
Figure 6.
Segments from AFM amplitude images showing cell cytoskeleton along the cell edges for (a) native, (b) DTF, (c) IL1β and (d) IL1β and DTF treated cells. Dominant and well-defined cytoskeletal fibers are observed at cell edges for all DTF treated cells, which are absent in their unstretched counterparts. (e) Expression of β-actin protein (top lane) and mRNA (middle lane) in control untreated chondrocytes, or chondrocytes exposed to IL-1β (1 ng/ml), DTF (6% at 0.05 Hz), or IL-1β and DTF. Cells were treated for 12 h for protein or 4 h for mRNA analysis. GAPDH (Bottom lane) was used as an internal control.
DISCUSSION
Our investigations reveal that mechanical stress in the form of dynamic tensile forces (DTF) influences the cell-surface topography of native or IL1β-treated chondrocytes isolated from the superficial layer of cartilage. In particular, our AFM investigations have provided two novel insights: (a) treatment with DTF induces increased density and sizes of granular structures on the cell surface for both native and IL1β treated cells and (b) distribution of β-actin filaments is remodeled in response to DTF for both native and IL1β treated chondrocytes.
Several biochemical changes have been shown by us and others to result after subjecting the chondrocytes to mechanical strain or pro-inflammatory factors, Long (2007), Agarwal et al. (2006), Xu et al. (2000). Prolonged exposure (> 24 hrs) of IL-1β to chondrocytes as performed in our studies has been reported to enhance the expression of a number of cell-surface receptors like the urokinase-type plasminogen activator receptor (uPAR) and membrane bound matrix metalloproteases (MMPs), Schwab et. al. (2004), Lyons-Giordano et. al. (1991), Saas et. al. (2006), complement regulatory proteins (CRPs) CD46, CD55 and CD59 mRNA, Hyc et. al. (2003), chemokine receptor CCR-5, Yuan et. al. (2001), CD44 and the proteinase-activated receptors (PAR-2), Xiang et. al. (2006). Thus the increase in the size of the granules observed on our IL1β treated chondrocyte surface as compared to native cells could be explained by the increased expression of the above cell surface receptors and/or their complexes.
Mechanotransduction pathways on the other hand utilize cell-surface receptors like α5β1-integrin, discoidin domain receptor 2 (DDR2), Shyu et. al. (2005), Lam et al (2007) or stretch-activated ion channels (SAC), Lee et. al. (2000). Furthermore, mechanostimulation of chondrocytes also leads to reorganization of focal adhesion contacts, Takahasi et. al. (2003) or can stimulate matrix metabolism leading to increased aggrecan, glycosaminoglycans (GAGs) and collagen synthesis rate, Sharma et. al. (2007), Nugent et. al. (2006), Stoltz et. al. (2000), Guilak et. al. (2006). Thus, in our studies the increase in the number and size of granular structures on the cell surface by DTF may reflect the increased secretion of the pericellular matrix components, upregulation and/or clustering of cell-surface receptors or focal adhesion complexes. In our studies both DTF and IL1-β induced an increase in the average size of granular structures on the cell surface but only DTF could increase the density of granules present on the cell surface, suggesting that upregulation of cell-surface molecules by IL1β and DTF may be regulated differently and DTF can result in biochemical changes even despite the presence of IL-1β.
Further work involving specific labeling of cell-surface molecules coupled with AFM analysis would help ascertain the relative quantities and time course of expression of these surface complexes. AFM topographic imaging and surface roughness measurements have served as a valuable tool to quantify the distribution of specific membrane proteins on cell surfaces when labeled with antibodies, Kienberger et. al. (2006), such as, the cystic fibrosis transmembrane conductance regulator protein on erythrocyte membranes, Schillers et. al. (2007) and CD34, CD44 or CD29 antigens on the surfaces of adipose tissue derived mesenchymal stem cells (MSCs), Shu et. al. (2007). High-resolution AFM imaging coupled with AFM force spectroscopy can identify the exact location and distribution of cell-surface proteins such as Angiotensin II, Li G. et al (2005), Osteopontin, Ron et. al. (2007) and vascular endothelial growth factor (VEGF) receptor, Almqvist et. al. (2004). Our findings for the first time demonstrate that cultured chondrocytes are amenable to high-resolution AFM imaging and a quantitative analysis of the distribution of cell-surface molecules can provide new insights into the chondrocyte response to mechanical or inflammatory factors.
Chondrocytes sense and adapt to external mechanical stimuli through the actin cytoskeleton which also provides the cell its mechanical support and is linked to the focal adhesion complexes. For example, in response to changes in mechanical environment, actin together with talin, α-actinin, filamin, vinculin, and integrins undergoes reorganization and realignment at the cell surface, to allow communication with the extracellular matrix and thus to regulate cellular responses. Changes in the actin organization in chondrocytes have been reported after exposure to hypoosmotic stimulation or hydrostatic forces Guilak et. al. (2002), Chao et. al. (2006), Fioravanti et. al. (2003 & 2005) and Knight et al (2006). However, limited studies exist on the effect of mechanical strains at physiological levels on actin remodeling in chondrocytes. Campbell et al have demonstrated that, cyclic uniaxial compressive loading leads to net peripheral actin breakdown in articular chondrocytes with no significant change in expression of actin-binding proteins. In contrast, oscillatory tension causes an enhanced peripheral actin organization in cellular projections in chondrocytes, Vanderploeg et. al. (2004). Our results that DTF enhances peripheral actin remodeling support the hypothesis that tensile forces play an important role on the peripheral actin remodeling Vanderploeg et al (2004) and phenotypic differentiation of chondrocytes, Takahasi et. al. (2003). In both earlier, Knight et al. (2006) and our present studies, changes in actin organization were not associated with changes in actin gene expression suggesting that the response is due to a remodeling of existing actin.
It has been reported earlier that the expression and/or organization of cytoskeletal proteins, is affected by IL-1, Guilak et. al. (2002), Pritchard et. al. (2006), Vinal et al (2002). Further, non-OA chondrocytes are known to exhibit a higher elastic modulus and viscocity as compared to OA cells, Trickey et. al. (2004). In our observations chondrocytes cultured on collagen-coated flexible membranes, failed to show significant remodeling of actin cytoskeleton upon 24 hrs exposure to IL-1β as compared to native cells. One possible explanation could be the transient nature of IL-1β effects as suggested by Pritchard et. al. (2006), wherein IL-1β induced changes in actin can sensitize the cell to further IL-1 exposure through clustering and subsequent internalization of membrane receptors, Singh et. al.(1999). Nevertheless, our results demonstrate that DTF can induce enhanced peripheral actin remodeling even in the presence of proinflammatory stimulus, like IL1-β, suggesting that mechanical forces may play a crucial role in restoring chondrocyte stiffness and mechanotransduction properties in OA.
As discussed above, mechanical forces and proinflammatory mediators are known to differ significantly in inducing biochemical, cytoskeletal, and topographical changes on chondrocytes. While our earlier studies have elucidated certain biochemical changes induced by DTF on IL-1β treated cells, our current study revealed that prolonged exposure of chondrocytes to DTF in the physiological range may help overcome the catabolic effects of pro-inflammatory factors by restoring the chondrocyte mechanical properties and its capacity for cell-matrix interaction.
Acknowledgments
** This work was supported by AT00646 and DE015399
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal S, Deschner J, Long P, Verma A, Hofman C, Evans CH, Piesco N. Role of NF-kappaB transcription factors in antiinflammatory and proinflammatory actions of mechanical signals. Arthritis Rheum. 2004;50:3541–3548. doi: 10.1002/art.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Long PR, Gassner N, Piesco P, Buckley MJ. Cyclic tensile strain suppresses catabolic effects of interleukin-1beta in fibrochondrocytes from the temporomandibular joint. Arthritis Rheum. 2001;44:608–617. doi: 10.1002/1529-0131(200103)44:3<608::AID-ANR109>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almqvist N, Bhatia R, Primbs G, Desai N, Banerjee S, Lal R. Elasticity and adhesion force mapping reveals real-time clustering of growth factor receptors and associated changes in local cellular rheological properties. Biophys J. 2004;86(3):1753–1762. doi: 10.1016/S0006-3495(04)74243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ateshian GA. Artificial cartilage: weaving in three dimensions. Nat. Mater. 2007;6:89–90. doi: 10.1038/nmat1830. [DOI] [PubMed] [Google Scholar]
- Chao PH, West AC, Hung CT. Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. Am J Physiol Cell Physiol. 2006;291(4):C718–C725. doi: 10.1152/ajpcell.00127.2005. [DOI] [PubMed] [Google Scholar]
- Deschner J, Rath-Deschner B, Agarwal S. Regulation of matrix metalloproteinase expression by dynamic tensile strain in rat fibrochondrocytes Osteoarthritis Cartilage. 2006 Mar;14(3):264–272. doi: 10.1016/j.joca.2005.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti M, Gassner R, Wang Z, Perera P, Deschner J, Sowa G, Salter RB, Agarwal S. Biomechanical signals suppress proinflammatory responses in cartilage: early events in experimental antigen-induced arthritis. J. Immunol. 2006;177:8757–8766. doi: 10.4049/jimmunol.177.12.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravanti A, Benetti D, Coppola G, Collodel G. Effect of continuous high hydrostatic pressure on the morphology and cytoskeleton of normal and osteoarthritic human chondrocytes cultivated in alginate gels. Clin Exp Rheumatol. 2005;23(6):847–853. [PubMed] [Google Scholar]
- Fioravanti A, Nerucci F, Annefeld M, Collodel G, Marcolongo R. Morphological and cytoskeletal aspects of cultivated normal and osteoarthritic human articular chondrocytes after cyclical pressure: a pilot study. Clin Exp Rheumatol. 2003 Nov–Dec;21(6):739–746. [PubMed] [Google Scholar]
- Garcia AJ, Boettiger D. Integrin-fibronectin interactions at the cell-material interface: initial integrin binding and signaling. Biomaterials. 1999;20:2427–2433. doi: 10.1016/s0142-9612(99)00170-2. [DOI] [PubMed] [Google Scholar]
- Gassner RJ, Buckley MJ, Studer RK, Evans CH, Agarwal S. Interaction of strain and interleukin-1 in articular cartilage: effects on proteoglycan synthesis in chondrocytes. Int. J. Oral Maxillofac. Surg. 2000;29:389–394. [PMC free article] [PubMed] [Google Scholar]
- Goldmann WH. Mechanical aspects of cell shape regulation and signaling. Cell Biol. Int. 2002;26:313–317. doi: 10.1006/cbir.2002.0857. [DOI] [PubMed] [Google Scholar]
- Guilak F, A Leonidas G Alexopoulos, Maureen L Upton, A Inchan Youn, A Jae Bong Choi, Li Cao, A Lori A Setton, A And Mansoor A Haider. The Pericellular Matrix As A Transducer of Biomechanical and Biochemical Signals in Articular Cartilage Ann. N.Y. Acad. Sci. 2006;1068:498–512. doi: 10.1196/annals.1346.011. [DOI] [PubMed] [Google Scholar]
- Guilak F, Erickson GR, Ting-Beall HP. The effects of osmotic stress on the viscoelastic and physical properties of articular chondrocytes. Biophys J. 2002;82(2):720–727. doi: 10.1016/S0006-3495(02)75434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh MH, Nguyen HT. Molecular mechanism of apoptosis induced by mechanical forces. Int. Rev. Cytol. 2005;245:45–90. doi: 10.1016/S0074-7696(05)45003-2. [DOI] [PubMed] [Google Scholar]
- Hyc A, Osiecka-Iwan A, Strzelczyk P, Moskalewski S. Effect of IL-1beta, TNF-alpha and IL-4 on complement regulatory protein mRNA expression in human articular chondrocytes. Int J Mol Med. 2003;11(1):91–94. [PubMed] [Google Scholar]
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- Ji H, Pettit A, Ohmura K, Ortiz-Lopez A, Duchatelle V, Degott C, Gravallese E, Mathis D, Benoist C. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J. Exp Med. 2002;196(1):77–85. doi: 10.1084/jem.20020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J, Calabrese L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2004;43(Suppl 3):iii2–iii9. doi: 10.1093/rheumatology/keh201. [DOI] [PubMed] [Google Scholar]
- Kienberger F, Ebner A, Gruber HJ, Hinterdorfer P. Molecular recognition imaging and force spectroscopy of single biomolecules. Acc Chem Res. 2006;39(1):29–36. doi: 10.1021/ar050084m. [DOI] [PubMed] [Google Scholar]
- Knight MM, Toyoda T, Lee DA, Bader DL. Mechanical compression and hydrostatic pressure induce reversible changes in actin cytoskeletal organisation in chondrocytes in agarose. J Biomech. 2006;39(8):1547–1551. doi: 10.1016/j.jbiomech.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Lam NP, Li Y, Waldman AB, Brussiau J, Lee PL, Olsen BR, Xu L. Age-dependent increase of discoidin domain receptor 2 and matrix metalloproteinase 13 expression in temporomandibular joint cartilage of type IX and type XI collagen-deficient mice. Arch Oral Biol. 2007;52(6):579–584. doi: 10.1016/j.archoralbio.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Millward-Sadler SJ, Wright MO, Nuki G, Salter DM. Integrin and mechanosensitive ion channel-dependent tyrosine phosphorylation of focal adhesion proteins and beta -catenin in human articular chondrocytes after mechanical stimulation. J Bone Miner Res. 2000;(8):1501–1509. doi: 10.1359/jbmr.2000.15.8.1501. [DOI] [PubMed] [Google Scholar]
- LeGrand A, Fermor B, Fink C, Pisetsky DS, Weinberg JB, Vail TP, Guilak F. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum. 2001;44(9):2078–2083. doi: 10.1002/1529-0131(200109)44:9<2078::AID-ART358>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Li G, Xi N, Wang DH. Investigation of angiotensin II type 1 receptor by atomic force microscopy with functionalized tip. Nanomedicine. 2005 Dec;1(4):306–312. doi: 10.1016/j.nano.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Long P, Gassner R, Agarwal S. Tumor necrosis factor alpha-dependent proinflammatory gene induction is inhibited by cyclic tensile strain in articular chondrocytes in vitro. Arth & Rheumat. 2001;44:2311–2319. doi: 10.1002/1529-0131(200110)44:10<2311::aid-art393>3.0.co;2-q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons-Giordano B, Kefalides NA, Brinker JM, Pratta MA, Arner EC. The effects of interleukin-1 on the expression of thrombospondin and fibronectin by rabbit articular chondrocytes. Exp Cell Res. 1991 Aug;195(2):462–467. doi: 10.1016/0014-4827(91)90397-d. [DOI] [PubMed] [Google Scholar]
- Madhavan S, Anghelina M, Sjostrom D, Dossumbekova A, Guttridge DC, Agarwal S. Biomechanical signals suppress TAK1 activation to inhibit NF-kappaB transcriptional activation in fibrochondrocytes. J Immunol. 2007 Nov 1;179(9):6246–6254. doi: 10.4049/jimmunol.179.9.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobasheri A, Carter SD, Martín-Vasallo P, Shakibaei M. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol Int. 2002;26(1):1–18. doi: 10.1006/cbir.2001.0826. [DOI] [PubMed] [Google Scholar]
- Moreland LW. Biologic therapies on the horizon for rheumatoid arthritis. J. Clin. Rheumatol. 2004;10:S32–S39. doi: 10.1097/01.rhu.0000130688.68036.ef. [DOI] [PubMed] [Google Scholar]
- Nugent GE, Schmidt TA, Schumacher BL, Voegtline MS, Bae WC, Jadin KD, Sah RL. Static and dynamic compression regulate cartilage metabolism of PRoteoGlycan 4 (PRG4) Biorheology. 2006;43(3–4):191–200. [PubMed] [Google Scholar]
- Nugent-Derfus GE, Takara T, O'neill JK, Cahill SB, Gortz S, Pong T, Inoue H, Aneloski NM, Wang WW, Vega KI, Klein TJ, Hsieh-Bonassera ND, Bae WC, Burke JD, Bugbee WD, Sah RL. Continuous passive motion applied to whole joints stimulates chondrocyte biosynthesis of PRG4. Osteoarthritis Cartilage. 2006 doi: 10.1016/j.joca.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard S, Guilak F. Effects of interleukin-1 on calcium signaling and the increase of filamentous actin in isolated and in situ articular chondrocytes. Arthritis & Rheumatism. 2006;54(7):2164–2174. doi: 10.1002/art.21941. [DOI] [PubMed] [Google Scholar]
- Renner AF, Carvalho E, Soares E, Mattiello-Rosa S. The effect of a passive muscle stretching protocol on the articular cartilage. Osteoarthritis Cartilage. 2006;14(2):196–202. doi: 10.1016/j.joca.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Ron A, Singh RR, Fishelson N, Socher R, Benayahu D, Shacham-Diamand Y. Site localization of membrane-bound proteins on whole cell level using atomic force microscopy. Biophys Chem. 2007 Nov 12; doi: 10.1016/j.bpc.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Saas J, Haag J, Rueger D, Chubinskaya S, Sohler F, Zimmer R, Bartnik E, Aigner T. IL-1beta, but not BMP-7 leads to a dramatic change in the gene expression pattern of human adult articular chondrocytes--portraying the gene expression pattern in two donors. Cytokine. 2006 Oct;36(1–2):90–99. doi: 10.1016/j.cyto.2006.10.005. Epub 2006. [DOI] [PubMed] [Google Scholar]
- Salter DM, Wright MO, Millward-Sadler SJ. NMDA receptor expression and roles in human articular chondrocyte mechanotransduction. Biorheology. 2004;41(3–4):273–281. [PubMed] [Google Scholar]
- Schillers H. Imaging CFTR in its native environment. Pflugers Arch. 2007 doi: 10.1007/s00424-007-0399-8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Schwab W, Schulze-Tanzil G, Mobasheri A, Dressler J, Kotzsch M, Shakibaei M. Interleukin-1beta-induced expression of the urokinase-type plasminogen activator receptor and its co-localization with MMPs in human articular chondrocytes. Histol Histopathol. 2004;19(1):105–112. doi: 10.14670/HH-19.105. [DOI] [PubMed] [Google Scholar]
- Sharma G, Saxena RK, Mishra P. Differential effects of cyclic and static pressure on biochemical and morphological properties of chondrocytes from articular cartilage. Clin Biomech. 2007;22(2):248–255. doi: 10.1016/j.clinbiomech.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Shu W, Shu YT, Shi HB, Fei LX, Lei W, Ming QG. An easy method to discover cell membrane antigen with atomic force microscopy. Mol Biol Rep. 2007 doi: 10.1007/s11033-007-9122-2. [DOI] [PubMed] [Google Scholar]
- Shyu KG, Chao YM, Wang BW, Kuan P. Regulation of discoidin domain receptor 2 by cyclic mechanical stretch in cultured rat vascular smooth muscle cells. Hypertension. 2005 Sep;46(3):614–621. doi: 10.1161/01.HYP.0000175811.79863.e2. [DOI] [PubMed] [Google Scholar]
- Singh R, Wang B, Shirvaikar A, Khan S, Kamat S, Schelling JR, et al. The IL-1 receptor and Rho directly associate to drive cell activation in inflammation. J Clin Invest. 1999;103:1561–1570. doi: 10.1172/JCI5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz JF, Dumas D, Wang X, Payan E, Mainard D, Paulus F, Maurice G, Netter P, Muller S. Influence of mechanical forces on cells and tissues. Biorheology. 2000;37(1–2):3–14. [PubMed] [Google Scholar]
- Strombeck B, Jacobsson LT. The role of exercise in the rehabilitation of patients with systemic lupus erythematosus and patients with primary sjogren's syndrome. Curr Opin Rheumatol. 2007;19(2):197–203. doi: 10.1097/BOR.0b013e32801494e3. [DOI] [PubMed] [Google Scholar]
- Takahashi I, Onodera K, Sasano Y, Mizoguchi I, Bae JW, Mitani H, Kagayama M, Mitani H. Effect of stretching on gene expression of beta1 integrin and focal adhesion kinase and on chondrogenesis through cell-extracellular matrix interactions. Eur J Cell Biol. 2003 Apr;82(4):182–192. doi: 10.1078/0171-9335-00307. [DOI] [PubMed] [Google Scholar]
- Trickey WR, Vail TP, Guilak F. The role of the cytoskeleton in the viscoelastic properties of human articular chondrocytes. J Orthop Res. 2004 Jan;22(1):131–139. doi: 10.1016/S0736-0266(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Vanderploeg EJ, Imler SM, Brodkin KR, García AJ, Levenston ME. Oscillatory tension differentially modulates matrix metabolism and cytoskeletal organization in chondrocytes and fibrochondrocytes. J Biomech. 2004 Dec;37(12):1941–1952. doi: 10.1016/j.jbiomech.2004.02.048. [DOI] [PubMed] [Google Scholar]
- Vinall RL, Lo SH, Reddi AH. Regulation of articular chondrocyte phenotype by bone morphogenetic protein 7, interleukin 1, and cellular context is dependent on the cytoskeleton. Exp Cell Res. 2002 Jan 1;272(1):32–44. doi: 10.1006/excr.2001.5395. [DOI] [PubMed] [Google Scholar]
- Weissmann G. The pathogenesis of rheumatoid arthritis. Bull. Hosp. Jt. Dis. 2006;64:12–15. [PubMed] [Google Scholar]
- Wolf A, Ackermann B, Steinmeyer J. Collagen synthesis of articular cartilage explants in response to frequency of cyclic mechanical loading. Cell Tissue Res. 2007;327(1):155–166. doi: 10.1007/s00441-006-0251-z. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Masuko-Hongo K, Sekine T, Nakamura H, Yudoh K, Nishioka K, Kato T. Expression of proteinase-activated receptors (PAR)-2 in articular chondrocytes is modulated by IL-1beta, TNF-alpha and TGF-beta. Osteoarthritis Cartilage. 2006 Nov;14(11):1163–1173. doi: 10.1016/j.joca.2006.04.015. Epub 2006 Jun 6. [DOI] [PubMed] [Google Scholar]
- Xu Z, Buckley MJ, Evans CH, Agarwal S. Cyclic tensile strain acts as an antagonist of IL-1 beta actions in chondrocytes. J Immunol. 2000;165(1):453–460. doi: 10.4049/jimmunol.165.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan GH, Masuko-Hongo K, Sakata M, Tsuruha J, Onuma H, Nakamura H, Aoki H, Kato T, Nishioka K. The role of C-C chemokines and their receptors in osteoarthritis. Arthritis Rheum. 2001 May;44(5):1056–1070. doi: 10.1002/1529-0131(200105)44:5<1056::AID-ANR186>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]