Abstract
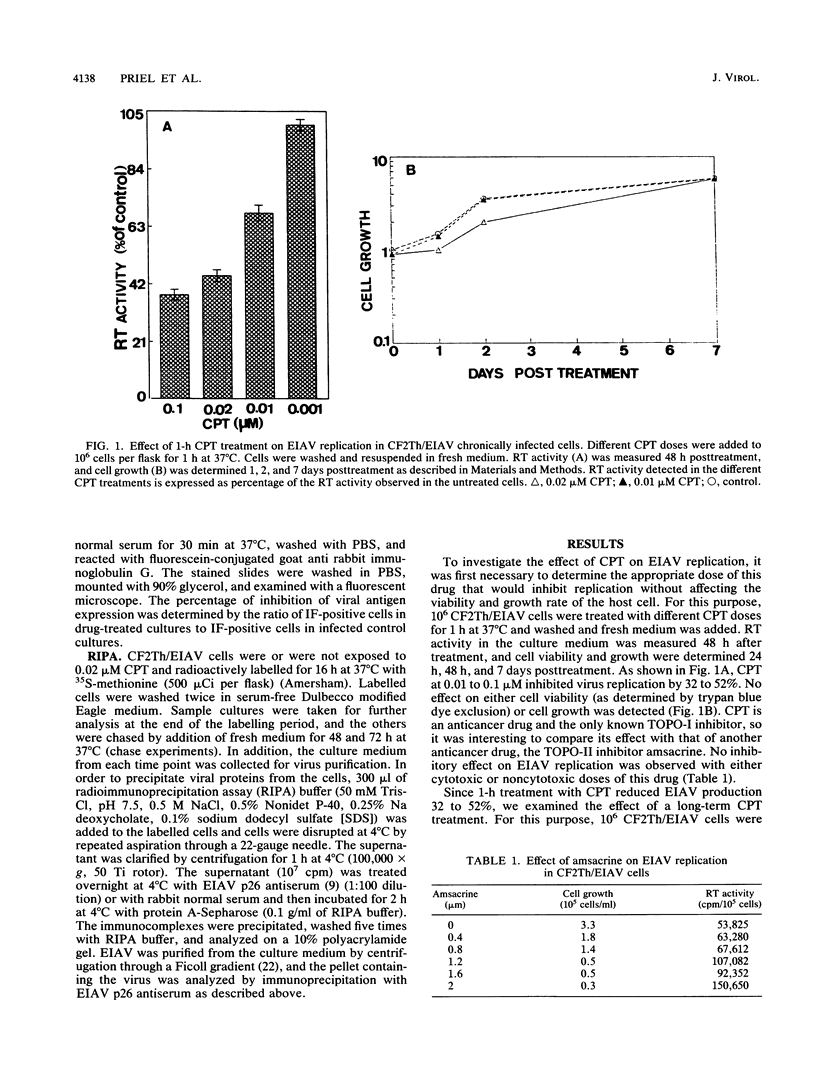
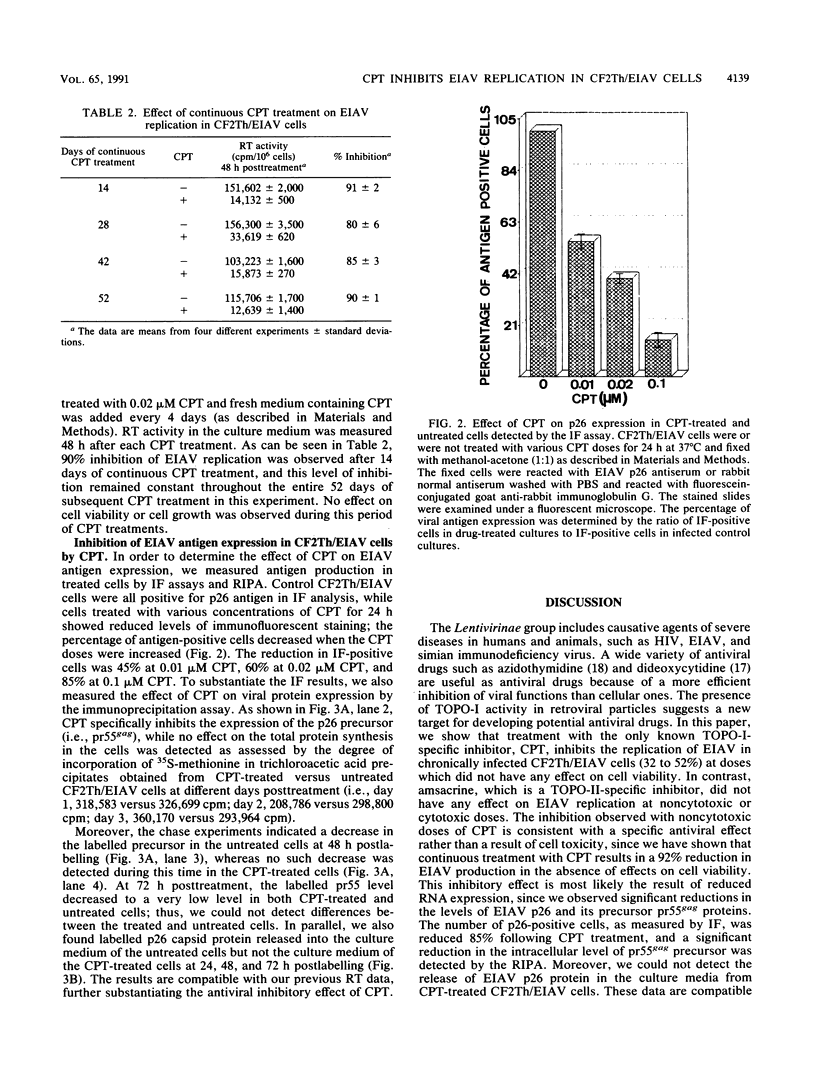
Camptothecin (CPT), a topoisomerase I-specific inhibitor, was found in this study to inhibit the replication of equine infectious anemia virus (EIAV) in chronically infected CF2Th cells (designated CF2Th/EIAV). By measuring viral reverse transcriptase activity in the culture medium, we demonstrated that treatment for 1 h with noncytotoxic doses of this drug inhibited production by 32 to 52%, whereas continuous exposure to this drug resulted in an 85 to 92% inhibition. No effect on the viability or growth rate of the cells was detected in any of these treatments. Indirect immunofluorescence analysis of the CPT-treated CF2Th/EIAV cells with anti-p26 capsid protein antibodies showed 60 to 85% reduction in the immunofluorescence-positive cells following drug treatment, and radioimmunoprecipitation analysis of these cells showed a comparable decrease of the pr55gag precursor protein. These data suggest that CPT acts as an anti-EIAV agent to block virus replication in the chronically infected cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andoh T., Ishii K., Suzuki Y., Ikegami Y., Kusunoki Y., Takemoto Y., Okada K. Characterization of a mammalian mutant with a camptothecin-resistant DNA topoisomerase I. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5565–5569. doi: 10.1073/pnas.84.16.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman H. P., Bladen S., Gilden R. V., Coggins L. Equine infectious anemia virus: evidence favoring classification as a retravirus. J Virol. 1976 Sep;19(3):1073–1079. doi: 10.1128/jvi.19.3.1073-1079.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu I. M., Yaniv A., Dahlberg J. E., Gazit A., Skuntz S. F., Tronick S. R., Aaronson S. A. Nucleotide sequence evidence for relationship of AIDS retrovirus to lentiviruses. 1985 Sep 26-Oct 2Nature. 317(6035):366–368. doi: 10.1038/317366a0. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Whang-Peng J., Adamson R. H. Studies on the antitumor activity, mechanism of action, and cell cycle effects of camptothecin. J Natl Cancer Inst. 1971 Apr;46(4):789–795. [PubMed] [Google Scholar]
- Giovanella B. C., Stehlin J. S., Wall M. E., Wani M. C., Nicholas A. W., Liu L. F., Silber R., Potmesil M. DNA topoisomerase I--targeted chemotherapy of human colon cancer in xenografts. Science. 1989 Nov 24;246(4933):1046–1048. doi: 10.1126/science.2555920. [DOI] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S., Gupta R., Eng B., Lock R. B., Ross W. E., Hertzberg R. P., Caranfa M. J., Johnson R. K. Camptothecin-resistant mutants of Chinese hamster ovary cells containing a resistant form of topoisomerase I. Cancer Res. 1988 Nov 15;48(22):6404–6410. [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R. C., Smythers G. W., Oroszlan S. Chemical and immunological characterizations of equine infectious anemia virus gag-encoded proteins. J Virol. 1987 Apr;61(4):1116–1124. doi: 10.1128/jvi.61.4.1116-1124.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., Hartshorn K. L., Rota T. R., Andrews C. A., Kaplan J. C., Schooley R. T., Hirsch M. S. Recombinant human interferon alfa-A suppresses HTLV-III replication in vitro. Lancet. 1985 Mar 16;1(8429):602–604. doi: 10.1016/s0140-6736(85)92144-0. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S., Horwitz S. B. Intracellular degradation of HeLa and adenovirus type 2 DNA induced by camptothecin. Biochem Biophys Res Commun. 1971 Nov 5;45(3):723–727. doi: 10.1016/0006-291x(71)90476-1. [DOI] [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Kono Y., Hirasawa K., Fukunaga Y., Taniguchi T. Recrudescence of equine infectious anemia by treatment with immunosuppressive drugs. Natl Inst Anim Health Q (Tokyo) 1976 Spring;16(1):8–15. [PubMed] [Google Scholar]
- McGuire T. C., Crawford T. B., Henson J. B. Immunofluorescent localization of equine infectious anemia virus in tissue. Am J Pathol. 1971 Feb;62(2):283–294. [PMC free article] [PubMed] [Google Scholar]
- McGuire T. C., Crawford T. B. Immunology of a persistent retrovirus infection--equine infectious anemia. Adv Vet Sci Comp Med. 1979;23:137–159. [PubMed] [Google Scholar]
- McGuire T. C., Henson J. B., Quist S. E. Viral-induced hemolysis in equine infectious anemia. Am J Vet Res. 1969 Dec;30(12):2091–2097. [PubMed] [Google Scholar]
- Mitsuya H., Broder S. Strategies for antiviral therapy in AIDS. 1987 Feb 26-Mar 4Nature. 325(6107):773–778. doi: 10.1038/325773a0. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Weinhold K. J., Furman P. A., St Clair M. H., Lehrman S. N., Gallo R. C., Bolognesi D., Barry D. W., Broder S. 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel E., Showalter S. D., Blair D. G. Inhibition of human immunodeficiency virus (HIV-1) replication in vitro by noncytotoxic doses of camptothecin, a topoisomerase I inhibitor. AIDS Res Hum Retroviruses. 1991 Jan;7(1):65–72. doi: 10.1089/aid.1991.7.65. [DOI] [PubMed] [Google Scholar]
- Priel E., Showalter S. D., Roberts M., Oroszlan S., Segal S., Aboud M., Blair D. G. Topoisomerase I activity associated with human immunodeficiency virus (HIV) particles and equine infectious anemia virus core. EMBO J. 1990 Dec;9(12):4167–4172. doi: 10.1002/j.1460-2075.1990.tb07640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. M., Oroszlan S. The preparation and biochemical characterization of intact capsids of equine infectious anemia virus. Biochem Biophys Res Commun. 1989 Apr 28;160(2):486–494. doi: 10.1016/0006-291x(89)92459-5. [DOI] [PubMed] [Google Scholar]
- Rushlow K., Olsen K., Stiegler G., Payne S. L., Montelaro R. C., Issel C. J. Lentivirus genomic organization: the complete nucleotide sequence of the env gene region of equine infectious anemia virus. Virology. 1986 Dec;155(2):309–321. doi: 10.1016/0042-6822(86)90195-9. [DOI] [PubMed] [Google Scholar]
- Spataro A., Kessel D. Studies on camptothecin-induced degradation and apparent reaggregation of DNA from L1210 cells. Biochem Biophys Res Commun. 1972 Aug 7;48(3):643–648. doi: 10.1016/0006-291x(72)90396-8. [DOI] [PubMed] [Google Scholar]
- Stephens R. M., Casey J. W., Rice N. R. Equine infectious anemia virus gag and pol genes: relatedness to visna and AIDS virus. Science. 1986 Feb 7;231(4738):589–594. doi: 10.1126/science.3003905. [DOI] [PubMed] [Google Scholar]



