Abstract
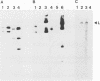
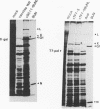
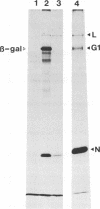
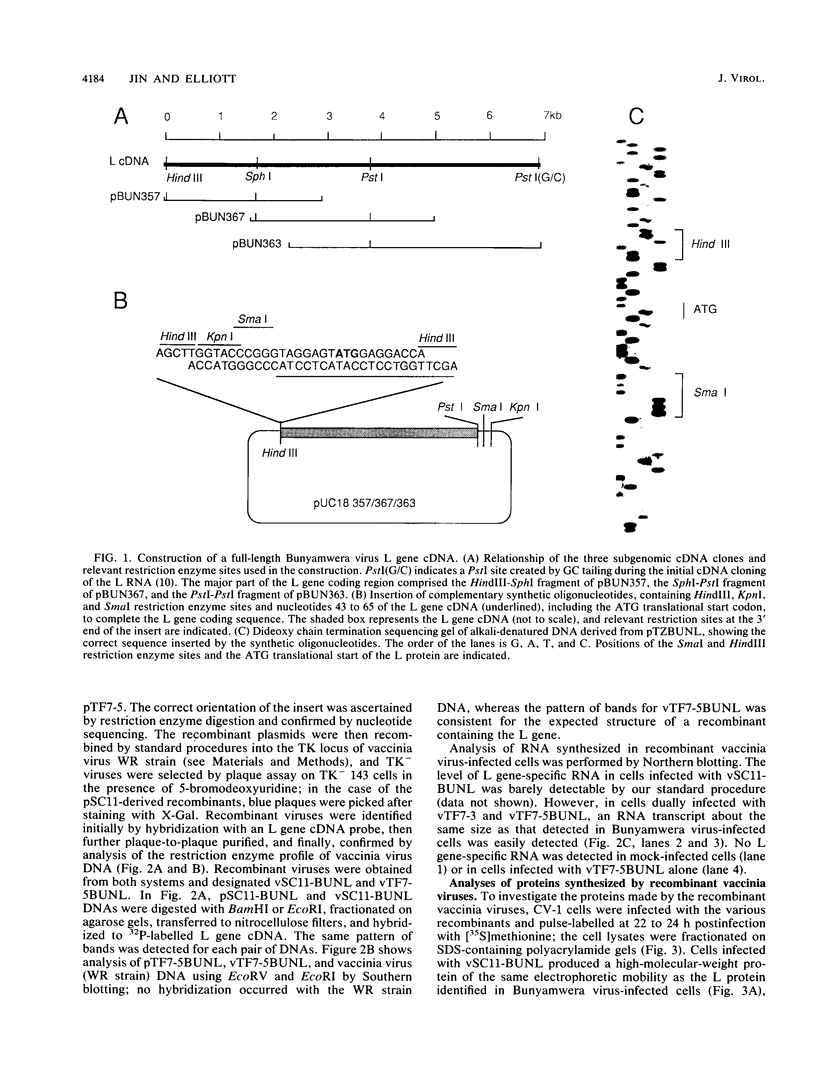
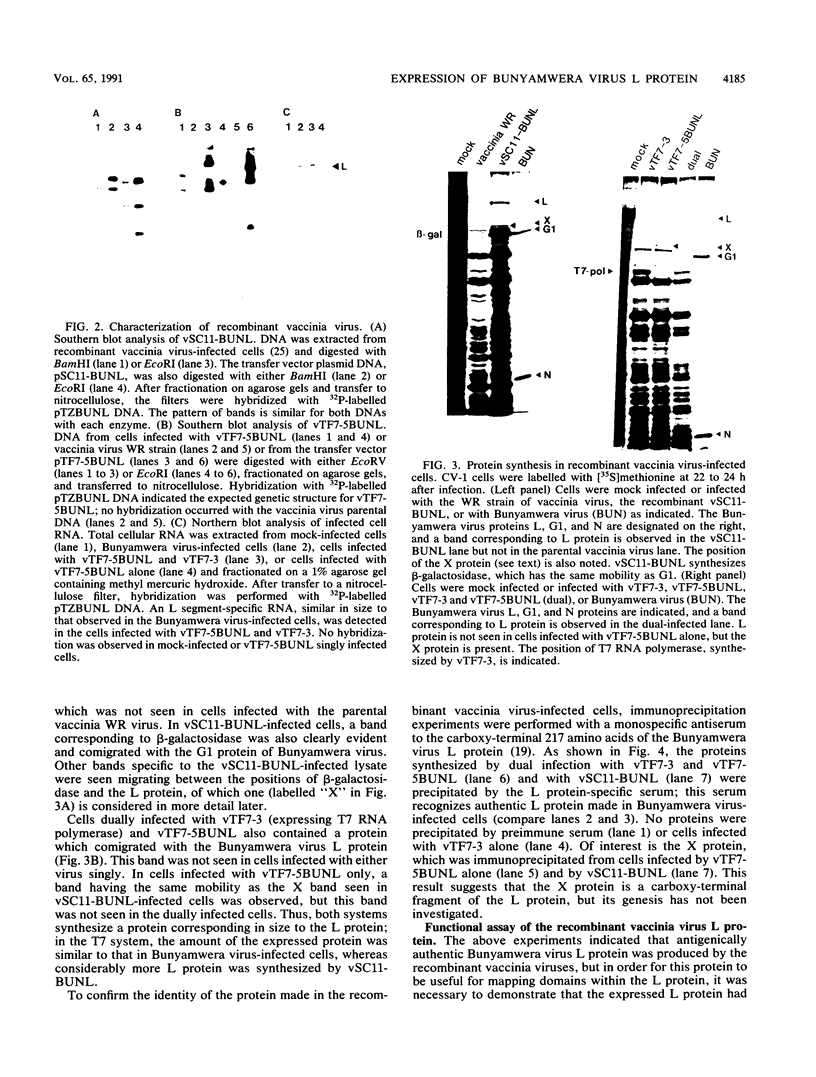
A cDNA containing the complete coding sequence of the Bunyamwera virus (family Bunyaviridae) L genome segment has been constructed and cloned into two recombinant vaccinia virus expression systems. In the first, the L gene is under control of vaccinia virus P7.5 promoter; in the second, the L gene is under control of the bacteriophage T7 phi 10 promoter, and expression of the L gene requires coinfection with a second recombinant vaccinia virus which synthesizes T7 RNA polymerase. Both systems express a protein which is the same size as the Bunyamwera virus L protein and is recognized by a monospecific L antiserum. The expressed L protein was shown to be functional in synthesizing Bunyamwera virus RNA in a nucleocapsid transfection assay: recombinant vaccinia virus-infected cells were transfected with purified Bunyamwera virus nucleocapsids, and subsequently, total cellular RNA was analyzed by Northern (RNA) blotting. No Bunyamwera virus RNA was detected in control transfections, but in cells which had previously been infected with recombinant vaccinia viruses expressing the L protein, both positive- and negative-sense Bunyamwera virus S segment RNA was detected. The suitability of this system to delineate functional domains within the Bunyamwera virus L protein is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Gay M. E., Matsuoko Y. Nonviral heterogeneous sequences are present at the 5' ends of one species of snowshoe hare bunyavirus S complementary RNA. Nucleic Acids Res. 1983 Sep 24;11(18):6409–6418. doi: 10.1093/nar/11.18.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Hannoun C. Studies on lumbo virus replication. I. RNA-dependent RNA polymerase associated with virions. Virology. 1976 Jan;69(1):258–264. doi: 10.1016/0042-6822(76)90212-9. [DOI] [PubMed] [Google Scholar]
- Bouloy M., Pardigon N., Vialat P., Gerbaud S., Girard M. Characterization of the 5' and 3' ends of viral messenger RNAs isolated from BHK21 cells infected with Germiston virus (Bunyavirus). Virology. 1990 Mar;175(1):50–58. doi: 10.1016/0042-6822(90)90185-t. [DOI] [PubMed] [Google Scholar]
- Bressan G. M., Stanley K. K. pUEX, a bacterial expression vector related to pEX with universal host specificity. Nucleic Acids Res. 1987 Dec 10;15(23):10056–10056. doi: 10.1093/nar/15.23.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus early promoters. J Mol Biol. 1989 Dec 20;210(4):749–769. doi: 10.1016/0022-2836(89)90107-1. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus late promoters. J Mol Biol. 1989 Dec 20;210(4):771–784. doi: 10.1016/0022-2836(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Elliott R. M. Molecular biology of the Bunyaviridae. J Gen Virol. 1990 Mar;71(Pt 3):501–522. doi: 10.1099/0022-1317-71-3-501. [DOI] [PubMed] [Google Scholar]
- Elliott R. M. Nucleotide sequence analysis of the large (L) genomic RNA segment of Bunyamwera virus, the prototype of the family Bunyaviridae. Virology. 1989 Dec;173(2):426–436. doi: 10.1016/0042-6822(89)90555-2. [DOI] [PubMed] [Google Scholar]
- Elliott R. M. Nucleotide sequence analysis of the small (S) RNA segment of Bunyamwera virus, the prototype of the family Bunyaviridae. J Gen Virol. 1989 May;70(Pt 5):1281–1285. doi: 10.1099/0022-1317-70-5-1281. [DOI] [PubMed] [Google Scholar]
- Eshita Y., Ericson B., Romanowski V., Bishop D. H. Analyses of the mRNA transcription processes of snowshoe hare bunyavirus S and M RNA species. J Virol. 1985 Sep;55(3):681–689. doi: 10.1128/jvi.55.3.681-689.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Earl P. L., Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987 Jul;7(7):2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Moss B. Structure and stability of mRNA synthesized by vaccinia virus-encoded bacteriophage T7 RNA polymerase in mammalian cells. Importance of the 5' untranslated leader. J Mol Biol. 1989 Mar 20;206(2):333–348. doi: 10.1016/0022-2836(89)90483-x. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh H., Shioda T., Sakai Y., Mizumoto K., Shibuta H. Rescue of Sendai virus from viral ribonucleoprotein-transfected cells by infection with recombinant vaccinia viruses carrying Sendai virus L and P/C genes. Virology. 1989 Aug;171(2):434–443. doi: 10.1016/0042-6822(89)90612-0. [DOI] [PubMed] [Google Scholar]
- Hacker D., Rochat S., Kolakofsky D. Anti-mRNAs in La Crosse bunyavirus-infected cells. J Virol. 1990 Oct;64(10):5051–5057. doi: 10.1128/jvi.64.10.5051-5057.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D., Bellocq C., Raju R. The translational requirement for La Crosse virus S-mRNA synthesis. Cold Spring Harb Symp Quant Biol. 1987;52:373–379. doi: 10.1101/sqb.1987.052.01.043. [DOI] [PubMed] [Google Scholar]
- Krug R. M., St Angelo C., Broni B., Shapiro G. Transcription and replication of influenza virion RNA in the nucleus of infected cells. Cold Spring Harb Symp Quant Biol. 1987;52:353–358. doi: 10.1101/sqb.1987.052.01.040. [DOI] [PubMed] [Google Scholar]
- Lees J. F., Pringle C. R., Elliott R. M. Nucleotide sequence of the Bunyamwera virus M RNA segment: conservation of structural features in the Bunyavirus glycoprotein gene product. Virology. 1986 Jan 15;148(1):1–14. doi: 10.1016/0042-6822(86)90398-3. [DOI] [PubMed] [Google Scholar]
- Leppert M., Rittenhouse L., Perrault J., Summers D. F., Kolakofsky D. Plus and minus strand leader RNAs in negative strand virus-infected cells. Cell. 1979 Nov;18(3):735–747. doi: 10.1016/0092-8674(79)90127-2. [DOI] [PubMed] [Google Scholar]
- Li Y., Luo L. Z., Snyder R. M., Wagner R. R. Expression of the M gene of vesicular stomatitis virus cloned in various vaccinia virus vectors. J Virol. 1988 Mar;62(3):776–782. doi: 10.1128/jvi.62.3.776-782.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. L., Holloway B., Kolakofsky D. La Crosse virions contain a primer-stimulated RNA polymerase and a methylated cap-dependent endonuclease. J Virol. 1984 Oct;52(1):215–222. doi: 10.1128/jvi.52.1.215-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. L., Kolakofsky D. Characterization of La Crosse virus small-genome transcripts. J Virol. 1984 Mar;49(3):680–685. doi: 10.1128/jvi.49.3.680-685.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch O., Sauvaget I., Delarue M., Tordo N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989 Dec 1;8(12):3867–3874. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. Unusual transcripts in La Crosse virus-infected cells and the site for nucleocapsid assembly. J Virol. 1987 Mar;61(3):667–672. doi: 10.1128/jvi.61.3.667-672.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier C., Patterson J., Kolakofsky D. La Crosse virus small genome mRNA is made in the cytoplasm. J Virol. 1986 May;58(2):647–650. doi: 10.1128/jvi.58.2.647-650.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezza A. C., Repik P. M., Cash P., Bishop D. H. In vivo transcription and protein synthesis capabilities of bunyaviruses: wild-type snowshoe hare virus and its temperature-sensitive group I, group II, and group I/II mutants. J Virol. 1979 Aug;31(2):426–436. doi: 10.1128/jvi.31.2.426-436.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watret G. E., Pringle C. R., Elliott R. M. Synthesis of bunyavirus-specific proteins in a continuous cell line (XTC-2) derived from Xenopus laevis. J Gen Virol. 1985 Mar;66(Pt 3):473–482. doi: 10.1099/0022-1317-66-3-473. [DOI] [PubMed] [Google Scholar]