Abstract
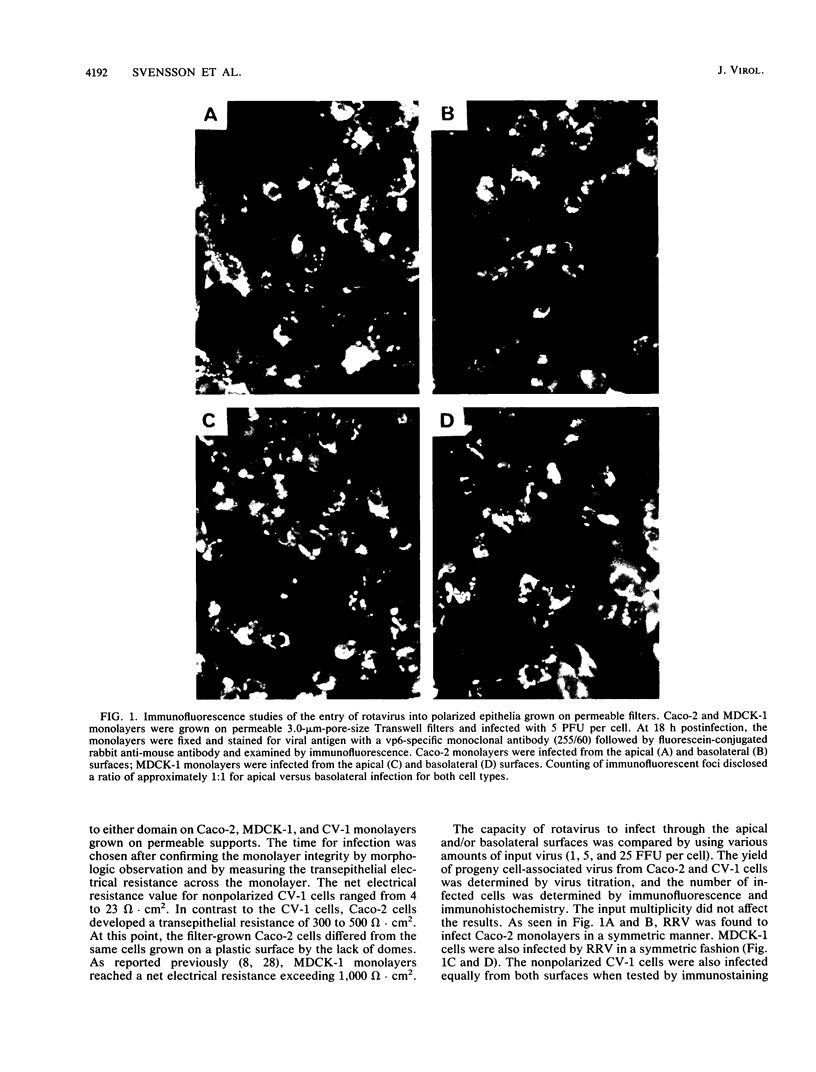
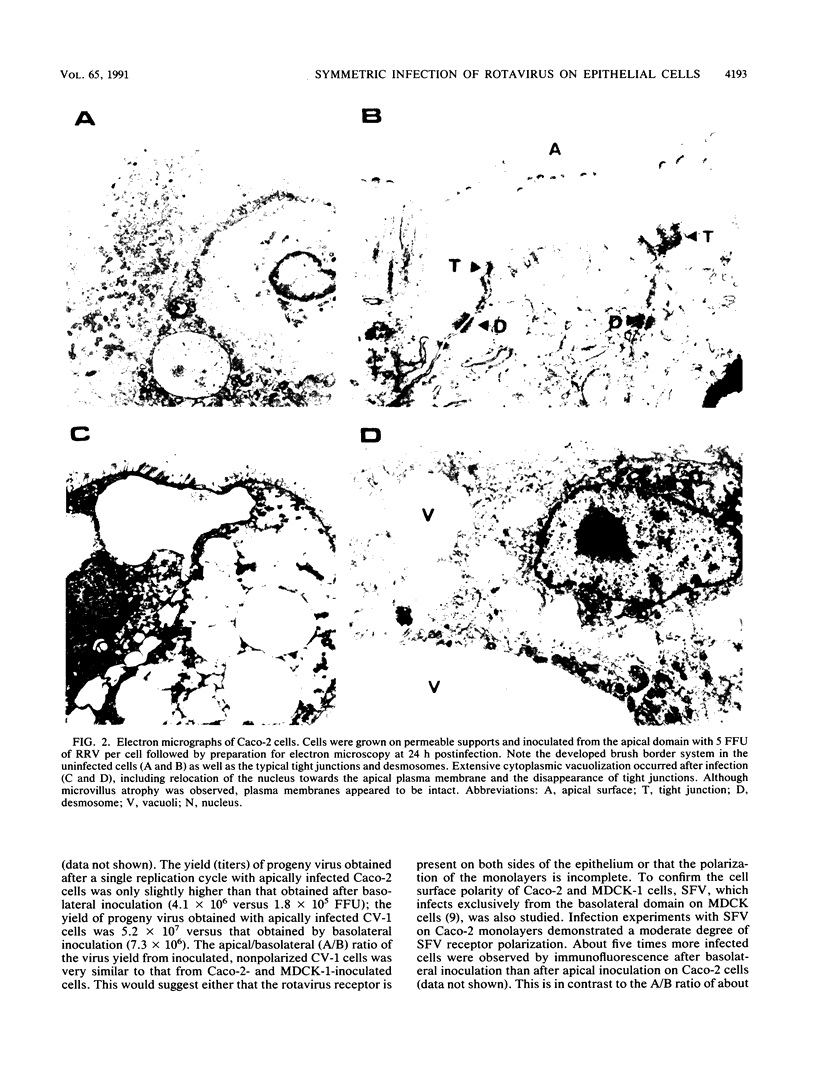
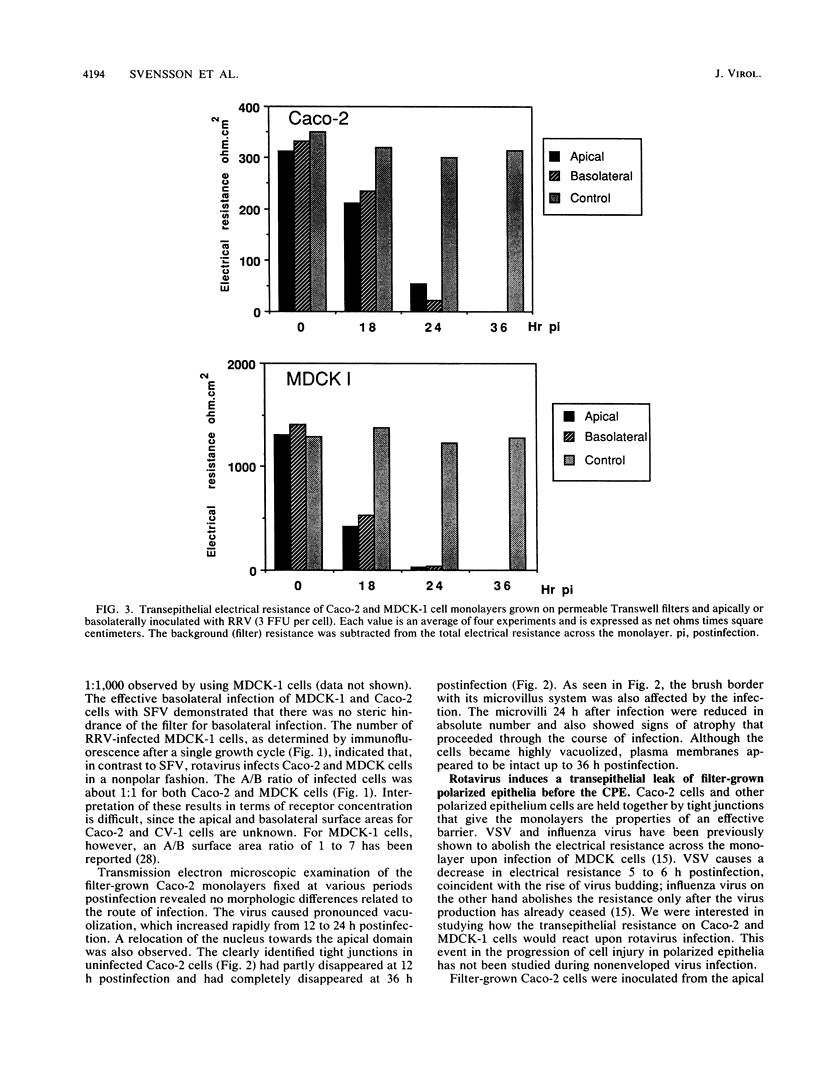
When rotavirus infects the mature villus tip cells of the small intestine, it encounters a highly polarized epithelium. In order to understand this virus-cell interaction more completely, we utilized a cell culture-adapted rhesus rotavirus (RRV) to infect human intestinal (Caco-2) and Madin-Darby canine kidney (MDCK-1) polarized epithelial cells grown on a permeable support. Filter-grown Caco-2 cells and MDCK-1 cells, producing a transepithelial resistance of 300 to 500 and greater than 1,000 omega . cm2, respectively, were infected from either the apical or basolateral domain with RRV or Semliki Forest virus. Whereas Semliki Forest virus infection only occurred when input virions had access to the basolateral domain of MDCK-1 or Caco-2 cells, RRV infected MDCK-1 and Caco-2 monolayers in a symmetric manner. The effect of rotavirus infection on monolayer permeability was analyzed by measuring the transepithelial electrical resistance. Rotavirus infection on filter-grown Caco-2 cells caused a transmembrane leak at 18 h postinfection, before the development of the cytopathic effect (CPE) and extensive virus release. Electrical resistance was completely abolished between 24 and 36 h postinfection. Although no CPE could be detected on RRV-infected MDCK cells, the infection caused a transmembrane leak that totally abolished the electrical resistance at 18 to 24 h postinfection. Cell viability and the CPE analysis together with immunohistochemistry and immunofluorescence data indicated that the abolishment of resistance across the monolayer was due not to an effect on the plasma membrane of the cells but to an effect on the paracellular pathway limited by tight junctions. Attachment and penetration of rotavirus onto Caco-2 cells caused no measurable transmembrane leak during the first hour of infection.
Full text
PDF


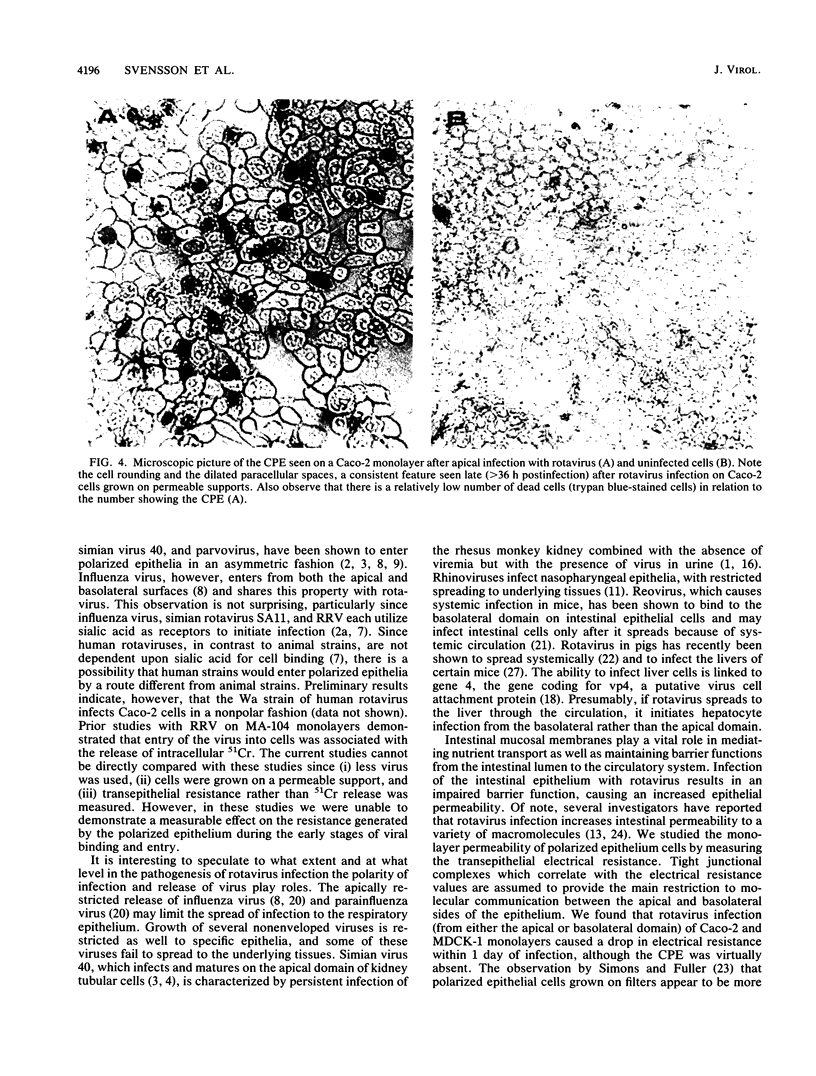

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHKENAZI A., MELNICK J. L. Induced latent infection of monkeys with vacuolating SV-40 Papova virus. Virus in kidneys and urine. Proc Soc Exp Biol Med. 1962 Nov;111:367–372. doi: 10.3181/00379727-111-27794. [DOI] [PubMed] [Google Scholar]
- Basak S., Compans R. W. Polarized entry of canine parvovirus in an epithelial cell line. J Virol. 1989 Jul;63(7):3164–3167. doi: 10.1128/jvi.63.7.3164-3167.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson E. T., Brando L. V., Compans R. W. Release of simian virus 40 virions from epithelial cells is polarized and occurs without cell lysis. J Virol. 1989 May;63(5):2278–2288. doi: 10.1128/jvi.63.5.2278-2288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson E. T., Compans R. W. Entry of simian virus 40 is restricted to apical surfaces of polarized epithelial cells. Mol Cell Biol. 1988 Aug;8(8):3391–3396. doi: 10.1128/mcb.8.8.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G. P., Goller I., Bishop R. F., Townley R. R., Holmes I. H., Ruck B. J. Immunofluorescence in duodenal mucosa of children with acute enteritis due to a new virus. J Clin Pathol. 1975 Apr;28(4):263–266. doi: 10.1136/jcp.28.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Gumbiner B., Falkow S. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J Cell Biol. 1988 Jul;107(1):221–230. doi: 10.1083/jcb.107.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukudome K., Yoshie O., Konno T. Comparison of human, simian, and bovine rotaviruses for requirement of sialic acid in hemagglutination and cell adsorption. Virology. 1989 Sep;172(1):196–205. doi: 10.1016/0042-6822(89)90121-9. [DOI] [PubMed] [Google Scholar]
- Fuller S. D., von Bonsdorff C. H., Simons K. Cell surface influenza haemagglutinin can mediate infection by other animal viruses. EMBO J. 1985 Oct;4(10):2475–2485. doi: 10.1002/j.1460-2075.1985.tb03959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S., von Bonsdorff C. H., Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984 Aug;38(1):65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- Gwaltney J. M., Jr Rhinoviruses. Yale J Biol Med. 1975 Mar;48(1):17–45. [PMC free article] [PubMed] [Google Scholar]
- Hidalgo I. J., Raub T. J., Borchardt R. T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989 Mar;96(3):736–749. [PubMed] [Google Scholar]
- Johansen K., Stintzing G., Magnusson K. E., Sundqvist T., Jalil F., Murtaza A., Khan S. R., Lindblad B. S., Möllby R., Orusild E. Intestinal permeability assessed with polyethylene glycols in children with diarrhea due to rotavirus and common bacterial pathogens in a developing community. J Pediatr Gastroenterol Nutr. 1989 Oct;9(3):307–313. doi: 10.1097/00005176-198910000-00008. [DOI] [PubMed] [Google Scholar]
- Kaljot K. T., Shaw R. D., Rubin D. H., Greenberg H. B. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J Virol. 1988 Apr;62(4):1136–1144. doi: 10.1128/jvi.62.4.1136-1144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Vancell R., Beaty G., Stefani E., Rodríguez-Boulan E. E., Cereijido M. Changes in paracellular and cellular ionic permeabilities of monolayers of MDCK cells infected with influenza or vesicular stomatitis viruses. J Membr Biol. 1984;81(3):171–180. doi: 10.1007/BF01868711. [DOI] [PubMed] [Google Scholar]
- MEYER H. M., Jr, HOPPS H. E., ROGERS N. G., BROOKS B. E., BERNHEIM B. C., JONES W. P., NISALAK A., DOUGLAS R. D. Studies on simian virus 40. J Immunol. 1962 Jun;88:796–806. [PubMed] [Google Scholar]
- Ramig R. F., Galle K. L. Rotavirus genome segment 4 determines viral replication phenotype in cultured liver cells (HepG2). J Virol. 1990 Mar;64(3):1044–1049. doi: 10.1128/jvi.64.3.1044-1049.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Nelson W. J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989 Aug 18;245(4919):718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Rubin D. H. Reovirus serotype 1 binds to the basolateral membrane of intestinal epithelial cells. Microb Pathog. 1987 Sep;3(3):215–219. doi: 10.1016/0882-4010(87)90098-2. [DOI] [PubMed] [Google Scholar]
- Shaw D. P., Morehouse L. G., Solorzano R. F. Rotavirus replication in colostrum-fed and colostrum-deprived pigs. Am J Vet Res. 1989 Nov;50(11):1966–1970. [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kitaoka S., Konno T., Sato T., Ishida N. Two modes of human rotavirus entry into MA 104 cells. Arch Virol. 1985;85(1-2):25–34. doi: 10.1007/BF01317003. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Konno T. Reovirus-like particles in jejunal mucosa of a Japanese infant with acute infectious non-bacterial gastroenteritis. Tohoku J Exp Med. 1975 Mar;115(3):199–211. doi: 10.1620/tjem.115.199. [DOI] [PubMed] [Google Scholar]
- Uhnoo I., Riepenhoff-Talty M., Dharakul T., Chegas P., Fisher J. E., Greenberg H. B., Ogra P. L. Extramucosal spread and development of hepatitis in immunodeficient and normal mice infected with rhesus rotavirus. J Virol. 1990 Jan;64(1):361–368. doi: 10.1128/jvi.64.1.361-368.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Fuller S. D., Simons K. Apical and basolateral endocytosis in Madin-Darby canine kidney (MDCK) cells grown on nitrocellulose filters. EMBO J. 1985 Nov;4(11):2781–2792. doi: 10.1002/j.1460-2075.1985.tb04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]