Abstract
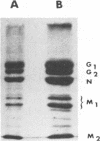
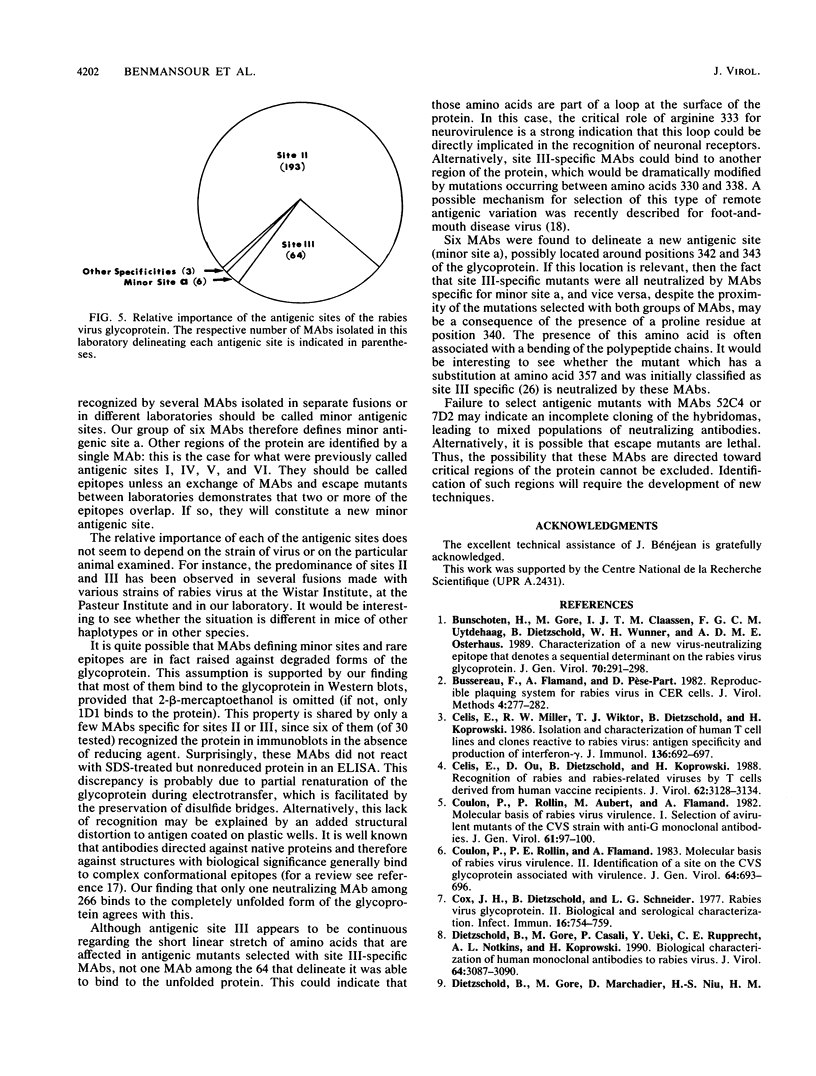
Although the number of antigenic sites on the rabies virus glycoprotein that have been described regularly increases with time, no attempt has been made to carefully evaluate the relative importance of each of these sites. Here we provide a more precise description of the antigenicity of the protein in mice of the H-2d haplotype; we developed this description by using 264 newly isolated monoclonal antibodies (MAbs) and a collection of neutralization-resistant (MAR) mutants. Most of the MAbs (97%) recognized antigenic sites previously described as II and III. One minor antigenic site separated from site III by three amino acids, including a proline, was identified (minor site a). Despite their proximity, there is no overlap between site III and minor site a; i.e., site III-specific MAR mutants were neutralized by the six MAbs defining minor site a, and vice versa. One of our MAbs, 1D1, reacted with sodium dodecyl sulfate-treated glycoprotein in Western blots (immunoblots) under reducing conditions and was therefore probably directed against a linear epitope, A MAR mutant selected with this MAb was still neutralized by MAbs of other specificities. This linear epitope was called G1 (G, Gif). As a general rule, we propose to reserve the term "antigenic site" (either major or minor) for regions of the protein which are defined by several MAbs originating from different fusions and to describe regions of the protein which are defined by a single MAb as epitopes. It would be interesting to test whether the same regions of the rabies virus glycoprotein are antigenic in mice of different haplotypes or in other species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunschoten H., Gore M., Claassen I. J., Uytdehaag F. G., Dietzschold B., Wunner W. H., Osterhaus A. D. Characterization of a new virus-neutralizing epitope that denotes a sequential determinant on the rabies virus glycoprotein. J Gen Virol. 1989 Feb;70(Pt 2):291–298. doi: 10.1099/0022-1317-70-2-291. [DOI] [PubMed] [Google Scholar]
- Bussereau F., Flamand A., Pese-Part D. Reproducible plaquing system for rabies virus in CER cells. J Virol Methods. 1982 May;4(4-5):277–282. doi: 10.1016/0166-0934(82)90074-x. [DOI] [PubMed] [Google Scholar]
- Celis E., Miller R. W., Wiktor T. J., Dietzschold B., Koprowski H. Isolation and characterization of human T cell lines and clones reactive to rabies virus: antigen specificity and production of interferon-gamma. J Immunol. 1986 Jan;136(2):692–697. [PubMed] [Google Scholar]
- Celis E., Ou D., Dietzschold B., Koprowski H. Recognition of rabies and rabies-related viruses by T cells derived from human vaccine recipients. J Virol. 1988 Sep;62(9):3128–3134. doi: 10.1128/jvi.62.9.3128-3134.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulon P., Rollin P. E., Flamand A. Molecular basis of rabies virus virulence. II. Identification of a site on the CVS glycoprotein associated with virulence. J Gen Virol. 1983 Mar;64(Pt 3):693–696. doi: 10.1099/0022-1317-64-3-693. [DOI] [PubMed] [Google Scholar]
- Coulon P., Rollin P., Aubert M., Flamand A. Molecular basis of rabies virus virulence. I. Selection of avirulent mutants of the CVS strain with anti-G monoclonal antibodies. J Gen Virol. 1982 Jul;61(Pt 50):97–100. doi: 10.1099/0022-1317-61-1-97. [DOI] [PubMed] [Google Scholar]
- Cox J. H., Dietzschold B., Schneider L. G. Rabies virus glycoprotein. II. Biological and serological characterization. Infect Immun. 1977 Jun;16(3):754–759. doi: 10.1128/iai.16.3.754-759.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Gore M., Casali P., Ueki Y., Rupprecht C. E., Notkins A. L., Koprowski H. Biological characterization of human monoclonal antibodies to rabies virus. J Virol. 1990 Jun;64(6):3087–3090. doi: 10.1128/jvi.64.6.3087-3090.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Gore M., Marchadier D., Niu H. S., Bunschoten H. M., Otvos L., Jr, Wunner W. H., Ertl H. C., Osterhaus A. D., Koprowski H. Structural and immunological characterization of a linear virus-neutralizing epitope of the rabies virus glycoprotein and its possible use in a synthetic vaccine. J Virol. 1990 Aug;64(8):3804–3809. doi: 10.1128/jvi.64.8.3804-3809.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Wunner W. H., Wiktor T. J., Lopes A. D., Lafon M., Smith C. L., Koprowski H. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc Natl Acad Sci U S A. 1983 Jan;80(1):70–74. doi: 10.1073/pnas.80.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand A., Wiktor T. J., Koprowski H. Use of hybridoma monoclonal antibodies in the detection of antigenic differences between rabies and rabies-related virus proteins. II. The glycoprotein. J Gen Virol. 1980 May;48(1):105–109. doi: 10.1099/0022-1317-48-1-105. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafon M., Edelman L., Bouvet J. P., Lafage M., Montchâtre E. Human monoclonal antibodies specific for the rabies virus glycoprotein and N protein. J Gen Virol. 1990 Aug;71(Pt 8):1689–1696. doi: 10.1099/0022-1317-71-8-1689. [DOI] [PubMed] [Google Scholar]
- Lafon M., Ideler J., Wunner W. H. Investigation of the antigenic structure of rabies virus glycoprotein by monoclonal antibodies. Dev Biol Stand. 1984;57:219–225. [PubMed] [Google Scholar]
- Lafon M., Wiktor T. J., Macfarlan R. I. Antigenic sites on the CVS rabies virus glycoprotein: analysis with monoclonal antibodies. J Gen Virol. 1983 Apr;64(Pt 4):843–851. doi: 10.1099/0022-1317-64-4-843. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Smith-Gill S. J. Epitopes on protein antigens: misconceptions and realities. Cell. 1990 May 18;61(4):553–556. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- Parry N., Fox G., Rowlands D., Brown F., Fry E., Acharya R., Logan D., Stuart D. Structural and serological evidence for a novel mechanism of antigenic variation in foot-and-mouth disease virus. Nature. 1990 Oct 11;347(6293):569–572. doi: 10.1038/347569a0. [DOI] [PubMed] [Google Scholar]
- Prehaud C., Coulon P., LaFay F., Thiers C., Flamand A. Antigenic site II of the rabies virus glycoprotein: structure and role in viral virulence. J Virol. 1988 Jan;62(1):1–7. doi: 10.1128/jvi.62.1.1-7.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Préhaud C., Coulon P., Diallo A., Martinet-Edelist C., Flamand A. Characterization of a new temperature-sensitive and avirulent mutant of the rabies virus. J Gen Virol. 1989 Jan;70(Pt 1):133–143. doi: 10.1099/0022-1317-70-1-133. [DOI] [PubMed] [Google Scholar]
- Seif I., Coulon P., Rollin P. E., Flamand A. Rabies virulence: effect on pathogenicity and sequence characterization of rabies virus mutations affecting antigenic site III of the glycoprotein. J Virol. 1985 Mar;53(3):926–934. doi: 10.1128/jvi.53.3.926-934.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., György E., Schlumberger D., Sokol F., Koprowski H. Antigenic properties of rabies virus components. J Immunol. 1973 Jan;110(1):269–276. [PubMed] [Google Scholar]
- Wunner W. H., Dietzschold B. Rabies virus infection: genetic mutations and the impact on viral pathogenicity and immunity. Contrib Microbiol Immunol. 1987;8:103–124. [PubMed] [Google Scholar]
- Wunner W. H., Dietzschold B., Smith C. L., Lafon M., Golub E. Antigenic variants of CVS rabies virus with altered glycosylation sites. Virology. 1985 Jan 15;140(1):1–12. doi: 10.1016/0042-6822(85)90440-4. [DOI] [PubMed] [Google Scholar]