Abstract
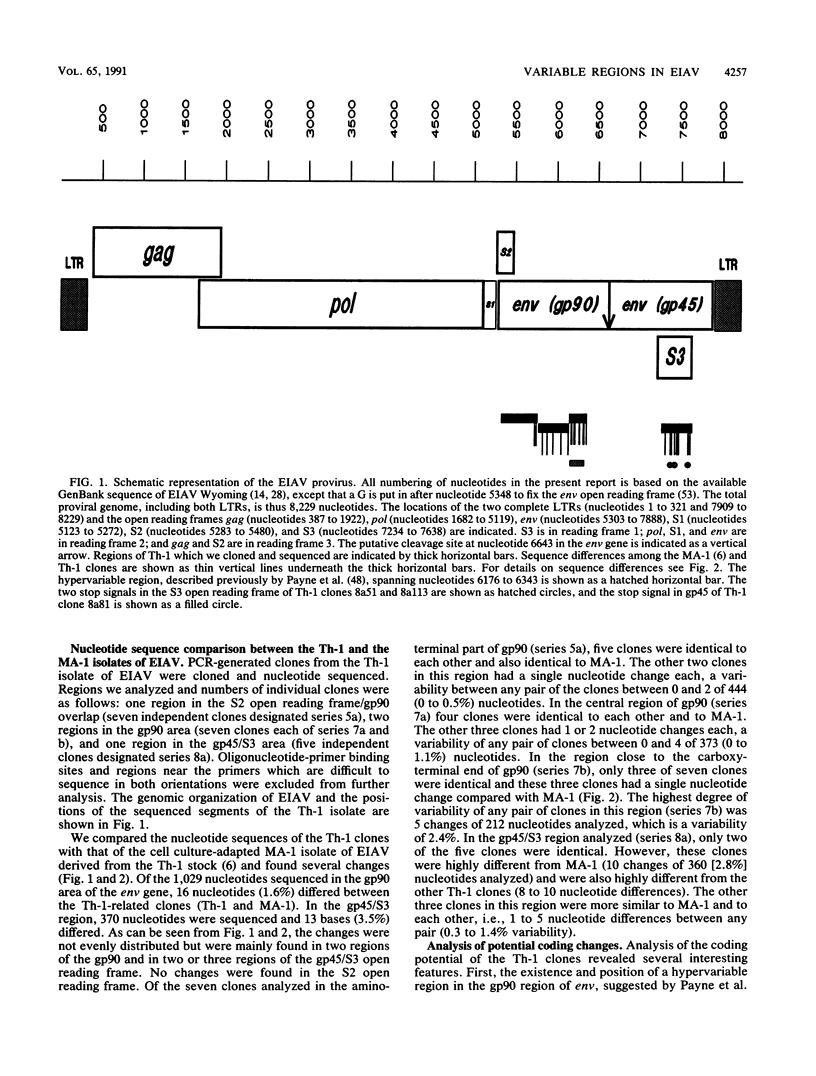
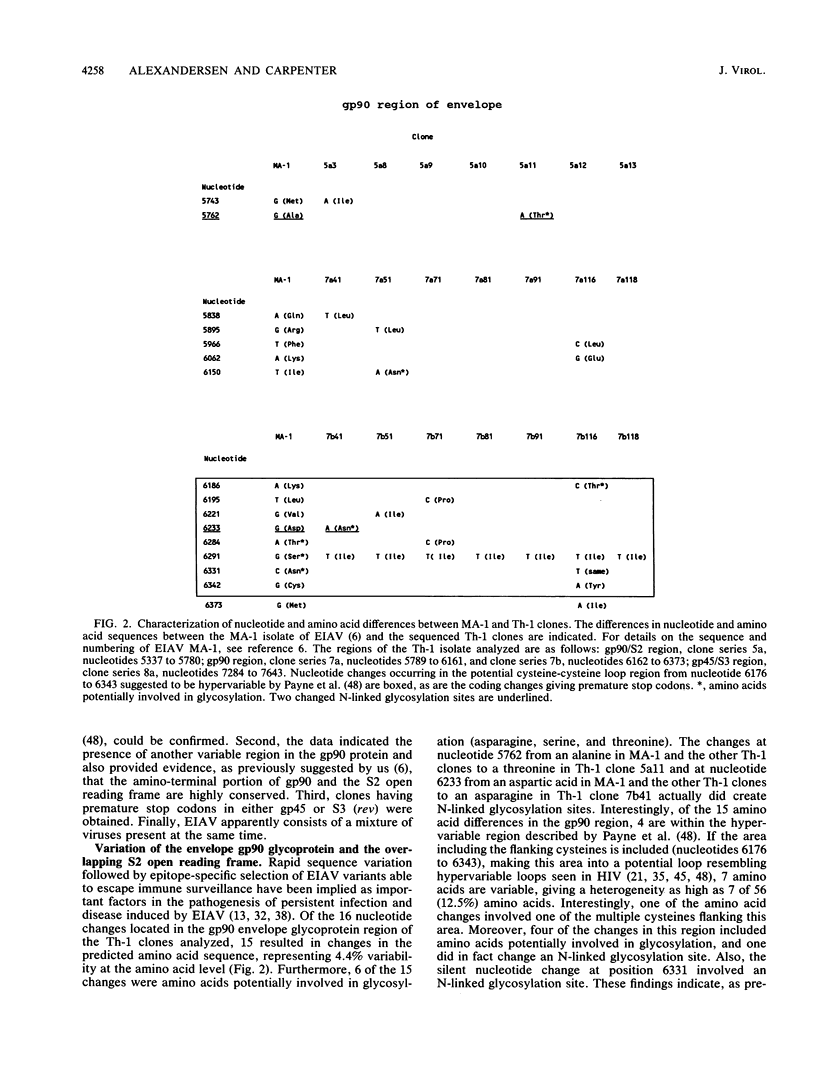
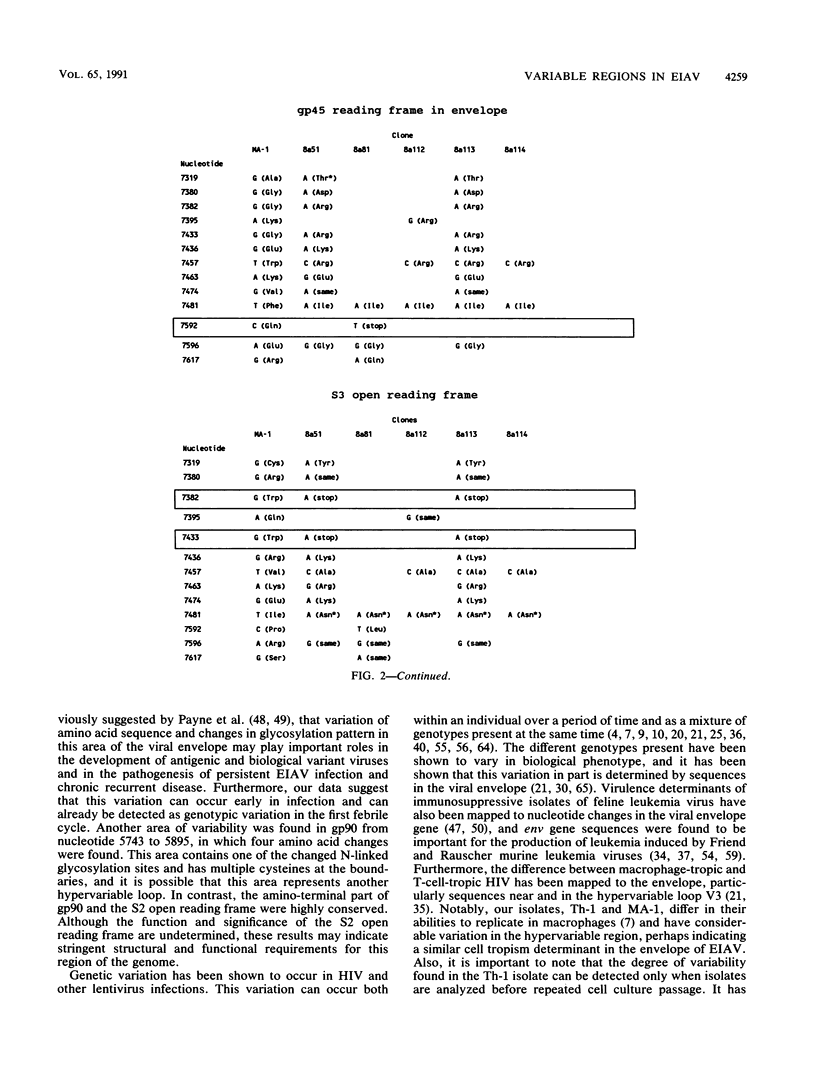
The polymerase chain reaction was used to amplify and clone parts of the envelope gene and overlapping S3 open reading frame, thought to encode rev, of the virulent in vivo-derived Th-1 isolate of equine infectious anemia virus (EIAV). The results indicated that EIAV consists of a heterogeneous mixture of genotypes present at the first febrile cycle after initial infection. We showed that the Th-1 isolate apparently contains nondefective genotypes as well as types which have transmembrane protein truncations or are rev deficient. Furthermore, we could confirm the presence of a hypervariable region in the gp90 envelope glycoprotein. Taken together with earlier data on the heterogeneity of the regulatory motifs present in the long terminal repeat sequences of viruses from the same in vivo isolate (S. Carpenter, S. Alexandersen, M. J. Long, S. Perryman, and B. Chesebro, J. Virol. 65:1605-1610, 1991), our findings indicate that EIAV uses a complex system of diversity in biological phenotypes together with variation in regulatory and antigenic makeup to evade host response and to cause persistent infection and recurrent chronic disease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Y. F., Hanly S. M., Malim M. H., Cullen B. R., Greene W. C. Structure-function analyses of the HTLV-I Rex and HIV-1 Rev RNA response elements: insights into the mechanism of Rex and Rev action. Genes Dev. 1990 Jun;4(6):1014–1022. doi: 10.1101/gad.4.6.1014. [DOI] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Perryman S. Detailed transcription map of Aleutian mink disease parvovirus. J Virol. 1988 Oct;62(10):3684–3694. doi: 10.1128/jvi.62.10.3684-3694.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Asjö B., Morfeldt-Månson L., Albert J., Biberfeld G., Karlsson A., Lidman K., Fenyö E. M. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986 Sep 20;2(8508):660–662. [PubMed] [Google Scholar]
- Bloom M. E., Alexandersen S., Perryman S., Lechner D., Wolfinbarger J. B. Nucleotide sequence and genomic organization of Aleutian mink disease parvovirus (ADV): sequence comparisons between a nonpathogenic and a pathogenic strain of ADV. J Virol. 1988 Aug;62(8):2903–2915. doi: 10.1128/jvi.62.8.2903-2915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S., Alexandersen S., Long M. J., Perryman S., Chesebro B. Identification of a hypervariable region in the long terminal repeat of equine infectious anemia virus. J Virol. 1991 Mar;65(3):1605–1610. doi: 10.1128/jvi.65.3.1605-1610.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S., Chesebro B. Change in host cell tropism associated with in vitro replication of equine infectious anemia virus. J Virol. 1989 Jun;63(6):2492–2496. doi: 10.1128/jvi.63.6.2492-2496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S., Evans L. H., Sevoian M., Chesebro B. Role of the host immune response in selection of equine infectious anemia virus variants. J Virol. 1987 Dec;61(12):3783–3789. doi: 10.1128/jvi.61.12.3783-3789.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Seto D., Tateno M., Levy J. A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988 Apr 1;240(4848):80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- Chiodi F., Valentin A., Keys B., Schwartz S., Asjö B., Gartner S., Popovic M., Albert J., Sundqvist V. A., Fenyö E. M. Biological characterization of paired human immunodeficiency virus type 1 isolates from blood and cerebrospinal fluid. Virology. 1989 Nov;173(1):178–187. doi: 10.1016/0042-6822(89)90233-x. [DOI] [PubMed] [Google Scholar]
- Chiu I. M., Yaniv A., Dahlberg J. E., Gazit A., Skuntz S. F., Tronick S. R., Aaronson S. A. Nucleotide sequence evidence for relationship of AIDS retrovirus to lentiviruses. 1985 Sep 26-Oct 2Nature. 317(6035):366–368. doi: 10.1038/317366a0. [DOI] [PubMed] [Google Scholar]
- Clements J. E., D'Antonio N., Narayan O. Genomic changes associated with antigenic variation of visna virus. II. Common nucleotide sequence changes detected in variants from independent isolations. J Mol Biol. 1982 Jul 5;158(3):415–434. doi: 10.1016/0022-2836(82)90207-8. [DOI] [PubMed] [Google Scholar]
- Clements J. E., Gdovin S. L., Montelaro R. C., Narayan O. Antigenic variation in lentiviral diseases. Annu Rev Immunol. 1988;6:139–159. doi: 10.1146/annurev.iy.06.040188.001035. [DOI] [PubMed] [Google Scholar]
- Derse D., Dorn P. L., Levy L., Stephens R. M., Rice N. R., Casey J. W. Characterization of equine infectious anemia virus long terminal repeat. J Virol. 1987 Mar;61(3):743–747. doi: 10.1128/jvi.61.3.743-747.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn P., DaSilva L., Martarano L., Derse D. Equine infectious anemia virus tat: insights into the structure, function, and evolution of lentivirus trans-activator proteins. J Virol. 1990 Apr;64(4):1616–1624. doi: 10.1128/jvi.64.4.1616-1624.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh E. J., Maury W. J., Folks T. M., Fauci A. S., Rabson A. B. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans K. S., Carpenter S. L., Sevoian M. Detection of equine infectious anemia virus in horse leukocyte cultures derived from horses in various stages of equine infectious anemia viral infection. Am J Vet Res. 1984 Jan;45(1):20–25. [PubMed] [Google Scholar]
- Felber B. K., Drysdale C. M., Pavlakis G. N. Feedback regulation of human immunodeficiency virus type 1 expression by the Rev protein. J Virol. 1990 Aug;64(8):3734–3741. doi: 10.1128/jvi.64.8.3734-3741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felber B. K., Hadzopoulou-Cladaras M., Cladaras C., Copeland T., Pavlakis G. N. rev protein of human immunodeficiency virus type 1 affects the stability and transport of the viral mRNA. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1495–1499. doi: 10.1073/pnas.86.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenyö E. M., Morfeldt-Månson L., Chiodi F., Lind B., von Gegerfelt A., Albert J., Olausson E., Asjö B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988 Nov;62(11):4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. G., Ensoli B., Looney D., Rose A., Gallo R. C., Saag M. S., Shaw G. M., Hahn B. H., Wong-Staal F. Biologically diverse molecular variants within a single HIV-1 isolate. Nature. 1988 Aug 4;334(6181):444–447. doi: 10.1038/334444a0. [DOI] [PubMed] [Google Scholar]
- Franchini G., Bosch M. L. Genetic relatedness of the human immunodeficiency viruses type 1 and 2 (HIV-1, HIV-2) and the simian immunodeficiency virus (SIV). Ann N Y Acad Sci. 1989;554:81–87. doi: 10.1111/j.1749-6632.1989.tb22412.x. [DOI] [PubMed] [Google Scholar]
- Golub E. I., Li G. G., Volsky D. J. Differences in the basal activity of the long terminal repeat determine different replicative capacities of two closely related human immunodeficiency virus type 1 isolates. J Virol. 1990 Aug;64(8):3654–3660. doi: 10.1128/jvi.64.8.3654-3660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda M. A., Braun M. J., Clements J. E., Pyper J. M., Wong-Staal F., Gallo R. C., Gilden R. V. Human T-cell lymphotropic virus type III shares sequence homology with a family of pathogenic lentiviruses. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4007–4011. doi: 10.1073/pnas.83.11.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Taylor M. E., Redfield R. R., Markham P. D., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Parks E. S., Parks W. P. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science. 1986 Jun 20;232(4757):1548–1553. doi: 10.1126/science.3012778. [DOI] [PubMed] [Google Scholar]
- Hanly S. M., Rimsky L. T., Malim M. H., Kim J. H., Hauber J., Duc Dodon M., Le S. Y., Maizel J. V., Cullen B. R., Greene W. C. Comparative analysis of the HTLV-I Rex and HIV-1 Rev trans-regulatory proteins and their RNA response elements. Genes Dev. 1989 Oct;3(10):1534–1544. doi: 10.1101/gad.3.10.1534. [DOI] [PubMed] [Google Scholar]
- Issel C. J., Coggins L. Equine infectious anemia: current knowledge. J Am Vet Med Assoc. 1979 Apr 1;174(7):727–733. [PubMed] [Google Scholar]
- Kawakami T., Sherman L., Dahlberg J., Gazit A., Yaniv A., Tronick S. R., Aaronson S. A. Nucleotide sequence analysis of equine infectious anemia virus proviral DNA. Virology. 1987 Jun;158(2):300–312. doi: 10.1016/0042-6822(87)90202-9. [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Byrn R., Groopman J., Baltimore D. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J Virol. 1989 Sep;63(9):3708–3713. doi: 10.1128/jvi.63.9.3708-3713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Ikeuchi K., Groopman J., Baltimore D. Factors affecting cellular tropism of human immunodeficiency virus. J Virol. 1990 Nov;64(11):5600–5604. doi: 10.1128/jvi.64.11.5600-5604.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Wooley D. P., Naidu Y. M., Kestler H. W., 3rd, Daniel M. D., Li Y., Desrosiers R. C. Significance of premature stop codons in env of simian immunodeficiency virus. J Virol. 1989 Nov;63(11):4709–4714. doi: 10.1128/jvi.63.11.4709-4714.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y., Kobayashi K., Fukunaga Y. Antigenic drift of equine infectious anemia virus in chronically infected horses. Arch Gesamte Virusforsch. 1973;41(1):1–10. doi: 10.1007/BF01249923. [DOI] [PubMed] [Google Scholar]
- Li Y., Golemis E., Hartley J. W., Hopkins N. Disease specificity of nondefective Friend and Moloney murine leukemia viruses is controlled by a small number of nucleotides. J Virol. 1987 Mar;61(3):693–700. doi: 10.1128/jvi.61.3.693-700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linemeyer D. L., Menke J. G., Ruscetti S. K., Evans L. H., Scolnick E. M. Envelope gene sequences which encode the gp52 protein of spleen focus-forming virus are required for the induction of erythroid cell proliferation. J Virol. 1982 Jul;43(1):223–233. doi: 10.1128/jvi.43.1.223-233.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. Q., Wood C., Levy J. A., Cheng-Mayer C. The viral envelope gene is involved in macrophage tropism of a human immunodeficiency virus type 1 strain isolated from brain tissue. J Virol. 1990 Dec;64(12):6148–6153. doi: 10.1128/jvi.64.12.6148-6153.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutley R., Pétursson G., Pálsson P. A., Georgsson G., Klein J., Nathanson N. Antigenic drift in visna: virus variation during long-term infection of Icelandic sheep. J Gen Virol. 1983 Jul;64(Pt 7):1433–1440. doi: 10.1099/0022-1317-64-7-1433. [DOI] [PubMed] [Google Scholar]
- Machida C. A., Bestwick R. K., Kabat D. A weakly pathogenic Rauscher spleen focus-forming virus mutant that lacks the carboxyl-terminal membrane anchor of its envelope glycoprotein. J Virol. 1985 Mar;53(3):990–993. doi: 10.1128/jvi.53.3.990-993.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire T. C., O'Rourke K., Cheevers W. P. A review of antigenic variation by the equine infectious anemia virus. Contrib Microbiol Immunol. 1987;8:77–89. [PubMed] [Google Scholar]
- McNearney T., Westervelt P., Thielan B. J., Trowbridge D. B., Garcia J., Whittier R., Ratner L. Limited sequence heterogeneity among biologically distinct human immunodeficiency virus type 1 isolates from individuals involved in a clustered infectious outbreak. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1917–1921. doi: 10.1073/pnas.87.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Montelaro R. C., Robey W. G., West M. D., Issel C. J., Fischinger P. J. Characterization of the serological cross-reactivity between glycoproteins of the human immunodeficiency virus and equine infectious anaemia virus. J Gen Virol. 1988 Jul;69(Pt 7):1711–1717. doi: 10.1099/0022-1317-69-7-1711. [DOI] [PubMed] [Google Scholar]
- Montelaro R., Ball J., Rwambo P., Issel C. Antigenic variation during persistent lentivirus infections and its implications for vaccine development. Adv Exp Med Biol. 1989;251:251–272. doi: 10.1007/978-1-4757-2046-4_24. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Chase J. Antigenic shift of visna virus in persistently infected sheep. Science. 1977 Jul 22;197(4301):376–378. doi: 10.1126/science.195339. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Strick N. Confronting the hypervariability of an immunodominant epitope eliciting virus neutralizing antibodies from the envelope glycoprotein of the human immunodeficiency virus type 1 (HIV-1). Mol Immunol. 1990 Jun;27(6):539–549. doi: 10.1016/0161-5890(90)90073-9. [DOI] [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbaugh J., Donahue P. R., Quackenbush S. L., Hoover E. A., Mullins J. I. Molecular cloning of a feline leukemia virus that induces fatal immunodeficiency disease in cats. Science. 1988 Feb 19;239(4842):906–910. doi: 10.1126/science.2893454. [DOI] [PubMed] [Google Scholar]
- Payne S. L., Fang F. D., Liu C. P., Dhruva B. R., Rwambo P., Issel C. J., Montelaro R. C. Antigenic variation and lentivirus persistence: variations in envelope gene sequences during EIAV infection resemble changes reported for sequential isolates of HIV. Virology. 1987 Dec;161(2):321–331. doi: 10.1016/0042-6822(87)90124-3. [DOI] [PubMed] [Google Scholar]
- Payne S. L., Rushlow K., Dhruva B. R., Issel C. J., Montelaro R. C. Localization of conserved and variable antigenic domains of equine infectious anemia virus envelope glycoproteins using recombinant env-encoded protein fragments produced in Escherichia coli. Virology. 1989 Oct;172(2):609–615. doi: 10.1016/0042-6822(89)90203-1. [DOI] [PubMed] [Google Scholar]
- Poss M. L., Mullins J. I., Hoover E. A. Posttranslational modifications distinguish the envelope glycoprotein of the immunodeficiency disease-inducing feline leukemia virus retrovirus. J Virol. 1989 Jan;63(1):189–195. doi: 10.1128/jvi.63.1.189-195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasty S., Dhruva B. R., Schiltz R. L., Shih D. S., Issel C. J., Montelaro R. C. Proviral DNA integration and transcriptional patterns of equine infectious anemia virus during persistent and cytopathic infections. J Virol. 1990 Jan;64(1):86–95. doi: 10.1128/jvi.64.1.86-95.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., Henderson L. E., Sowder R. C., Copeland T. D., Oroszlan S., Edwards J. F. Synthesis and processing of the transmembrane envelope protein of equine infectious anemia virus. J Virol. 1990 Aug;64(8):3770–3778. doi: 10.1128/jvi.64.8.3770-3778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushlow K., Olsen K., Stiegler G., Payne S. L., Montelaro R. C., Issel C. J. Lentivirus genomic organization: the complete nucleotide sequence of the env gene region of equine infectious anemia virus. Virology. 1986 Dec;155(2):309–321. doi: 10.1016/0042-6822(86)90195-9. [DOI] [PubMed] [Google Scholar]
- Ruta M., Bestwick R., Machida C., Kabat D. Loss of leukemogenicity caused by mutations in the membrane glycoprotein structural gene of Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4704–4708. doi: 10.1073/pnas.80.15.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag M. S., Hahn B. H., Gibbons J., Li Y., Parks E. S., Parks W. P., Shaw G. M. Extensive variation of human immunodeficiency virus type-1 in vivo. Nature. 1988 Aug 4;334(6181):440–444. doi: 10.1038/334440a0. [DOI] [PubMed] [Google Scholar]
- Sakai K., Dewhurst S., Ma X. Y., Volsky D. J. Differences in cytopathogenicity and host cell range among infectious molecular clones of human immunodeficiency virus type 1 simultaneously isolated from an individual. J Virol. 1988 Nov;62(11):4078–4085. doi: 10.1128/jvi.62.11.4078-4085.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short M. K., Okenquist S. A., Lenz J. Correlation of leukemogenic potential of murine retroviruses with transcriptional tissue preference of the viral long terminal repeats. J Virol. 1987 Apr;61(4):1067–1072. doi: 10.1128/jvi.61.4.1067-1072.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitbon M., Sola B., Evans L., Nishio J., Hayes S. F., Nathanson K., Garon C. F., Chesebro B. Hemolytic anemia and erythroleukemia, two distinct pathogenic effects of Friend MuLV: mapping of the effects to different regions of the viral genome. Cell. 1986 Dec 26;47(6):851–859. doi: 10.1016/0092-8674(86)90800-7. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Rosen C., Wong-Staal F., Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985 Jul 5;229(4708):74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Rosen C., Wong-Staal F., Salahuddin S. Z., Popovic M., Arya S., Gallo R. C., Haseltine W. A. Trans-acting transcriptional regulation of human T-cell leukemia virus type III long terminal repeat. Science. 1985 Jan 11;227(4683):171–173. doi: 10.1126/science.2981427. [DOI] [PubMed] [Google Scholar]
- Stephens R. M., Casey J. W., Rice N. R. Equine infectious anemia virus gag and pol genes: relatedness to visna and AIDS virus. Science. 1986 Feb 7;231(4738):589–594. doi: 10.1126/science.3003905. [DOI] [PubMed] [Google Scholar]
- Stephens R. M., Derse D., Rice N. R. Cloning and characterization of cDNAs encoding equine infectious anemia virus tat and putative Rev proteins. J Virol. 1990 Aug;64(8):3716–3725. doi: 10.1128/jvi.64.8.3716-3725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersmette M., Gruters R. A., de Wolf F., de Goede R. E., Lange J. M., Schellekens P. T., Goudsmit J., Huisman H. G., Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989 May;63(5):2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York-Higgins D., Cheng-Mayer C., Bauer D., Levy J. A., Dina D. Human immunodeficiency virus type 1 cellular host range, replication, and cytopathicity are linked to the envelope region of the viral genome. J Virol. 1990 Aug;64(8):4016–4020. doi: 10.1128/jvi.64.8.4016-4020.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


