Abstract
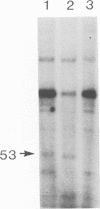
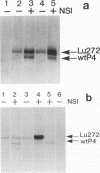
Parvovirus protein NS1 is required for replication of viral DNA and plays a role in the regulation of viral gene expression. NS1 trans-activates the P38 promoter for capsid protein synthesis and has variable effects on other promoters. In this study, we examined the effects of NS1 on the regulation of its own promoter, P4. A number of plasmid constructions were made with the P4 promoter fused to reporter genes. The effects of NS1 on expression from the P4 promoter differed depending on the construction. Plasmids containing viral sequences which could not replicate showed a decrease in P4 expression on cotransfection with the NS1 gene. However, plasmids having replication-proficient viral sequences showed a three- to fivefold increase in P4 expression dependent on NS1. The effect on NS1 on P4 transcription was also evaluated at the steady-state RNA level. An infectious clone of the LuIII viral genome was modified to an NS1-NS2 null mutant (pLu272) that is competent for viral DNA replication by introducing a frameshift mutation at codon 5 of the NS1 open reading frame. The P4 transcripts of pLu272 are four nucleotides longer than the wild type and can therefore be resolved from the wild type by primer extension analysis. pLu272 allows comparison of the constitutive level of steady-state RNA produced by the pLu272 P4 promoter in the absence or presence of a template replication dependent on NS1 supplied in trans. NS1 increased P4 transcripts about six- to eightfold. Expression of P4 transcripts from clones that could not amplify depended on the presence of an intact inverted terminal repeat sequence at the left end. A clone with an intact viral left end and a defective viral right end gave an NS1-dependent threefold increase in P4 expression. Destruction of terminal hairpins at both ends resulted in no significant increase in P4 expression in the presence of NS1. Thus, the positive effect of NS1 on the steady-state levels of P4 transcripts depends on the amplification of gene copy number and the integrity of the terminal repeats.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn J. K., Gavin B. J., Kumar G., Ward D. C. Transcriptional analysis of minute virus of mice P4 promoter mutants. J Virol. 1989 Dec;63(12):5425–5439. doi: 10.1128/jvi.63.12.5425-5439.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Astell C. R., Mol C. D., Anderson W. F. Structural and functional homology of parvovirus and papovavirus polypeptides. J Gen Virol. 1987 Mar;68(Pt 3):885–893. doi: 10.1099/0022-1317-68-3-885. [DOI] [PubMed] [Google Scholar]
- Beaton A., Palumbo P., Berns K. I. Expression from the adeno-associated virus p5 and p19 promoters is negatively regulated in trans by the rep protein. J Virol. 1989 Oct;63(10):4450–4454. doi: 10.1128/jvi.63.10.4450-4454.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell M. C., Astell C. R. A GC-box motif upstream of the B19 parvovirus unique promoter is important for in vitro transcription. J Virol. 1989 Nov;63(11):4814–4823. doi: 10.1128/jvi.63.11.4814-4823.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cornelis J. J., Chen Y. Q., Spruyt N., Duponchel N., Cotmore S. F., Tattersall P., Rommelaere J. Susceptibility of human cells to killing by the parvoviruses H-1 and minute virus of mice correlates with viral transcription. J Virol. 1990 Jun;64(6):2537–2544. doi: 10.1128/jvi.64.6.2537-2544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis J. J., Spruyt N., Spegelaere P., Guetta E., Darawshi T., Cotmore S. F., Tal J., Rommelaere J. Sensitization of transformed rat fibroblasts to killing by parvovirus minute virus of mice correlates with an increase in viral gene expression. J Virol. 1988 Sep;62(9):3438–3444. doi: 10.1128/jvi.62.9.3438-3444.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The NS-1 polypeptide of minute virus of mice is covalently attached to the 5' termini of duplex replicative-form DNA and progeny single strands. J Virol. 1988 Mar;62(3):851–860. doi: 10.1128/jvi.62.3.851-860.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Diffoot N., Shull B. C., Chen K. C., Stout E. R., Lederman M., Bates R. C. Identical ends are not required for the equal encapsidation of plus- and minus-strand parvovirus LuIII DNA. J Virol. 1989 Jul;63(7):3180–3184. doi: 10.1128/jvi.63.7.3180-3184.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerig C., Hirt B., Antonietti J. P., Beard P. Nonstructural protein of parvoviruses B19 and minute virus of mice controls transcription. J Virol. 1990 Jan;64(1):387–396. doi: 10.1128/jvi.64.1.387-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerig C., Hirt B., Beard P., Antonietti J. P. Minute virus of mice non-structural protein NS-1 is necessary and sufficient for trans-activation of the viral P39 promoter. J Gen Virol. 1988 Oct;69(Pt 10):2563–2573. doi: 10.1099/0022-1317-69-10-2563. [DOI] [PubMed] [Google Scholar]
- Hermonat P. L., Labow M. A., Wright R., Berns K. I., Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984 Aug;51(2):329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im D. S., Muzyczka N. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell. 1990 May 4;61(3):447–457. doi: 10.1016/0092-8674(90)90526-k. [DOI] [PubMed] [Google Scholar]
- Labow M. A., Hermonat P. L., Berns K. I. Positive and negative autoregulation of the adeno-associated virus type 2 genome. J Virol. 1986 Oct;60(1):251–258. doi: 10.1128/jvi.60.1.251-258.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Rhode S. L., 3rd Mutation of lysine 405 to serine in the parvovirus H-1 NS1 abolishes its functions for viral DNA replication, late promoter trans activation, and cytotoxicity. J Virol. 1990 Oct;64(10):4654–4660. doi: 10.1128/jvi.64.10.4654-4660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott S. J., Brady J. N. Enhancer function in viral and cellular gene regulation. Biochim Biophys Acta. 1989 Dec 17;989(2):97–110. doi: 10.1016/0304-419x(89)90037-1. [DOI] [PubMed] [Google Scholar]
- Merchlinsky M. J., Tattersall P. J., Leary J. J., Cotmore S. F., Gardiner E. M., Ward D. C. Construction of an infectious molecular clone of the autonomous parvovirus minute virus of mice. J Virol. 1983 Jul;47(1):227–232. doi: 10.1128/jvi.47.1.227-232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Kajigaya S., Shimada T., Young N. The gene encoding the nonstructural protein of B19 (human) parvovirus may be lethal in transfected cells. J Virol. 1988 Aug;62(8):2884–2889. doi: 10.1128/jvi.62.8.2884-2889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso P. R. Analysis of the protein-protein interactions in the parvovirus H-1 capsid. J Virol. 1983 Apr;46(1):94–102. doi: 10.1128/jvi.46.1.94-102.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Both excision and replication of cloned autonomous parvovirus DNA require the NS1 (rep) protein. J Virol. 1989 Oct;63(10):4249–4256. doi: 10.1128/jvi.63.10.4249-4256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Nucleotide sequence of the coat protein gene of canine parvovirus. J Virol. 1985 May;54(2):630–633. doi: 10.1128/jvi.54.2.630-633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus replication in normal and transformed human cells correlates with the nuclear translocation of the early protein NS1. J Virol. 1989 Jan;63(1):349–355. doi: 10.1128/jvi.63.1.349-355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. I. Kinetics in a parasynchronous cell system. J Virol. 1973 Jun;11(6):856–861. doi: 10.1128/jvi.11.6.856-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. III. Factors affecting H-1 RF DNA synthesis. J Virol. 1974 Oct;14(4):791–801. doi: 10.1128/jvi.14.4.791-801.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Richard S. M. Characterization of the trans-activation-responsive element of the parvovirus H-1 P38 promoter. J Virol. 1987 Sep;61(9):2807–2815. doi: 10.1128/jvi.61.9.2807-2815.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd trans-Activation of parvovirus P38 promoter by the 76K noncapsid protein. J Virol. 1985 Sep;55(3):886–889. doi: 10.1128/jvi.55.3.886-889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEIN H. M., ENDERS J. F. Multiplication and cytopathogenicity of Simian vacuolating virus 40 in cultures of human tissues. Proc Soc Exp Biol Med. 1962 Mar;109:495–500. doi: 10.3181/00379727-109-27246. [DOI] [PubMed] [Google Scholar]
- Samulski R. J., Chang L. S., Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J Virol. 1987 Oct;61(10):3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R. J., Srivastava A., Berns K. I., Muzyczka N. Rescue of adeno-associated virus from recombinant plasmids: gene correction within the terminal repeats of AAV. Cell. 1983 May;33(1):135–143. doi: 10.1016/0092-8674(83)90342-2. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Ehrbar M., zur Hausen H. Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helpervirus dependent parvovirus. Virology. 1986 Jul 15;152(1):110–117. doi: 10.1016/0042-6822(86)90376-4. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Heilbronn R., Georg-Fries B., zur Hausen H. Inhibition of initiator-induced SV40 gene amplification in SV40-transformed Chinese hamster cells by infection with a defective parvovirus. Int J Cancer. 1983 Nov 15;32(5):591–595. doi: 10.1002/ijc.2910320512. [DOI] [PubMed] [Google Scholar]
- Senapathy P., Tratschin J. D., Carter B. J. Replication of adeno-associated virus DNA. Complementation of naturally occurring rep- mutants by a wild-type genome or an ori- mutant and correction of terminal palindrome deletions. J Mol Biol. 1984 Oct 15;179(1):1–20. doi: 10.1016/0022-2836(84)90303-6. [DOI] [PubMed] [Google Scholar]
- Snyder R. O., Samulski R. J., Muzyczka N. In vitro resolution of covalently joined AAV chromosome ends. Cell. 1990 Jan 12;60(1):105–113. doi: 10.1016/0092-8674(90)90720-y. [DOI] [PubMed] [Google Scholar]
- Tattersall P. Replication of the parvovirus MVM. I. Dependence of virus multiplication and plaque formation on cell growth. J Virol. 1972 Oct;10(4):586–590. doi: 10.1128/jvi.10.4.586-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Layman K. R., Hand R. E. Effect of cell physiological state on infection by rat virus. J Virol. 1969 Dec;4(6):872–878. doi: 10.1128/jvi.4.6.872-878.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toolan H. W. The picodna viruses. H, RV, and AAV. Int Rev Exp Pathol. 1968;6:135–180. [PubMed] [Google Scholar]
- Tratschin J. D., Miller I. L., Carter B. J. Genetic analysis of adeno-associated virus: properties of deletion mutants constructed in vitro and evidence for an adeno-associated virus replication function. J Virol. 1984 Sep;51(3):611–619. doi: 10.1128/jvi.51.3.611-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., Tal J., Carter B. J. Negative and positive regulation in trans of gene expression from adeno-associated virus vectors in mammalian cells by a viral rep gene product. Mol Cell Biol. 1986 Aug;6(8):2884–2894. doi: 10.1128/mcb.6.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempe J. P., Carter B. J. Regulation of adeno-associated virus gene expression in 293 cells: control of mRNA abundance and translation. J Virol. 1988 Jan;62(1):68–74. doi: 10.1128/jvi.62.1.68-74.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter S., Richards R., Armentrout R. W. Cell cycle-dependent replication of the DNA of minute virus of mice, a parvovirus. Biochim Biophys Acta. 1980 May 30;607(3):420–431. doi: 10.1016/0005-2787(80)90152-5. [DOI] [PubMed] [Google Scholar]
- Yakobson B., Koch T., Winocour E. Replication of adeno-associated virus in synchronized cells without the addition of a helper virus. J Virol. 1987 Apr;61(4):972–981. doi: 10.1128/jvi.61.4.972-981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]