Abstract
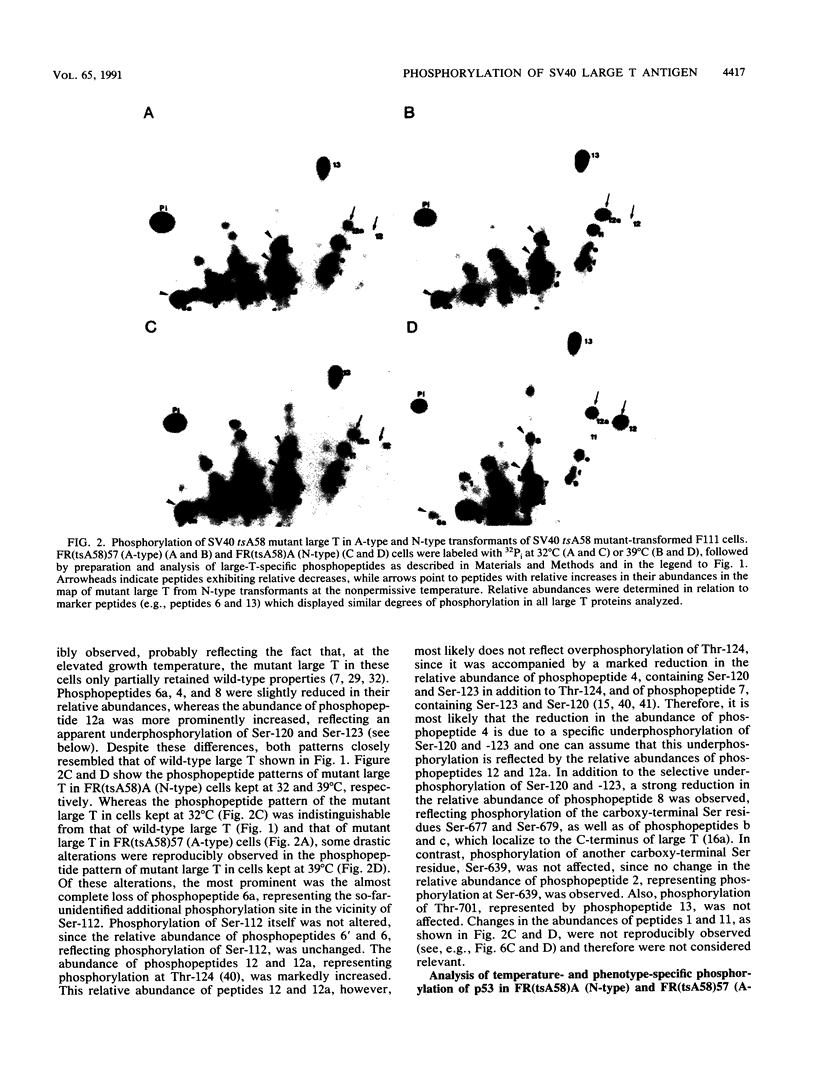
To identify molecular differences between simian virus 40 (SV40) tsA58 mutant large tumor antigen (large T) in cells of tsA58 N-type transformants [FR(tsA58)A cells], which revert to the normal phenotype after the cells are shifted to the nonpermissive growth temperature, and mutant large T in tsA58 A-type transformants [FR(tsA58)57 cells], which maintain their transformed phenotype after the temperature shift, we asked whether the biological activity of these mutant large T antigens at the nonpermissive growth temperature might correlate with phosphorylation at specific sites. At the permissive growth temperature, the phosphorylation patterns of the mutant large T proteins in FR(tsA58)A (N-type) cells and in FR(tsA58)57 (A-type) cells were largely indistinguishable from that of wild-type large T in FR(wt648) cells. After a shift to the nonpermissive growth temperature, no significant changes in the phosphorylation patterns of wild-type large T in FR(wt648) or of mutant large T in FR(tsA58)57 (A-type) cells were observed. In contrast, the phosphorylation pattern of mutant large T in FR(tsA58)A (N-type) cells changed in a characteristic manner, leading to an apparent underphosphorylation at specific sites. Phosphorylation of the cellular protein p53 was analyzed in parallel. Characteristic differences in the phosphorylation pattern of p53 were observed when cells of N-type and A-type transformants were kept at 39 degrees C as opposed to 32 degrees C. However, these differences did not relate to the different phenotypes of FR(tsA58)A (N-type) and FR(tsA58)57 (A-type) cells at the nonpermissive growth temperature. Our results, therefore, suggest that phosphorylation of large T at specific sites correlates with the transforming activity of tsA mutant large T in SV40 N-type and A-type transformants. This conclusion was substantiated by demonstrating that the biological properties as well as the phosphorylation patterns of SV40 tsA28 mutant large T in cells of SV40 tsA28 N-type and A-type transformants were similar to those in FR(tsA58)A (N-type) and in FR(tsA58)57 (A-type) cells, respectively. The phenotype-specific phosphorylation of tsA mutant large T in tsA A-type transformants probably is a cellular process induced during establishment of SV40 tsA A-type transformants, since tsA28 A-type transformant cells could be obtained by a large-T-dependent in vitro progression of cells of the tsA28 N-type transformant tsA28.3 (M. Osborn and K. Weber, J. Virol. 15:636-644, 1975).
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer M., Guhl E., Graessmann M., Graessmann A. Cellular mutation mediates T-antigen-positive revertant cells resistant to simian virus 40 transformation but not to retransformation by polyomavirus and adenovirus type 2. J Virol. 1987 Jun;61(6):1821–1827. doi: 10.1128/jvi.61.6.1821-1827.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourre F., Sarasin A. Targeted mutagenesis of SV40 DNA induced by UV light. Nature. 1983 Sep 1;305(5929):68–70. doi: 10.1038/305068a0. [DOI] [PubMed] [Google Scholar]
- Butel J. S., Jarvis D. L. The plasma-membrane-associated form of SV40 large tumor antigen: biochemical and biological properties. Biochim Biophys Acta. 1986 Oct 28;865(2):171–195. doi: 10.1016/0304-419x(86)90027-2. [DOI] [PubMed] [Google Scholar]
- Deppert W., Haug M. Evidence for free and metabolically stable p53 protein in nuclear subfractions of simian virus 40-transformed cells. Mol Cell Biol. 1986 Jun;6(6):2233–2240. doi: 10.1128/mcb.6.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Haug M., Steinmayer T. Modulation of p53 protein expression during cellular transformation with simian virus 40. Mol Cell Biol. 1987 Dec;7(12):4453–4463. doi: 10.1128/mcb.7.12.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W. SV40 T-antigen-related surface antigen: correlated expression with nuclear T-antigen in cells transformed by an SV40 A-gene mutant. Virology. 1980 Jul 30;104(2):497–501. doi: 10.1016/0042-6822(80)90353-0. [DOI] [PubMed] [Google Scholar]
- Deppert W., Steinmayer T., Richter W. Cooperation of SV40 large T antigen and the cellular protein p53 in maintenance of cell transformation. Oncogene. 1989 Sep;4(9):1103–1110. [PubMed] [Google Scholar]
- Freeman A. E., Igel H. J., Price P. J. I. In vitrol transformation of rat embryo cells: correlations with the known tumorigenic activities of chemicals in rodents. In Vitro. 1975 Mar-Apr;11(2):107–116. doi: 10.1007/BF02624083. [DOI] [PubMed] [Google Scholar]
- Gruss C., Wetzel E., Baack M., Mock U., Knippers R. High-affinity SV40 T-antigen binding sites in the human genome. Virology. 1988 Dec;167(2):349–360. [PubMed] [Google Scholar]
- Grässer F. A., Mann K., Walter G. Removal of serine phosphates from simian virus 40 large T antigen increases its ability to stimulate DNA replication in vitro but has no effect on ATPase and DNA binding. J Virol. 1987 Nov;61(11):3373–3380. doi: 10.1128/jvi.61.11.3373-3380.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Tamowski S., Deppert W. Antigenic binding sites of monoclonal antibodies specific for simian virus 40 large T antigen. J Virol. 1986 Mar;57(3):1168–1172. doi: 10.1128/jvi.57.3.1168-1172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinzpeter M., Deppert W. Analysis of biological and biochemical parameters for chromatin and nuclear matrix association of SV40 large T antigen in transformed cells. Oncogene. 1987 May;1(2):119–129. [PubMed] [Google Scholar]
- Hiscott J., Wong A., Alper D., Xanthoudakis S. trans activation of type 1 interferon promoters by simian virus 40 T antigen. Mol Cell Biol. 1988 Aug;8(8):3397–3405. doi: 10.1128/mcb.8.8.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höss A., Moarefi I., Scheidtmann K. H., Cisek L. J., Corden J. L., Dornreiter I., Arthur A. K., Fanning E. Altered phosphorylation pattern of simian virus 40 T antigen expressed in insect cells by using a baculovirus vector. J Virol. 1990 Oct;64(10):4799–4807. doi: 10.1128/jvi.64.10.4799-4807.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausing K., Scheidtmann K. H., Baumann E. A., Knippers R. Effects of in vitro dephosphorylation on DNA-binding and DNA helicase activities of simian virus 40 large tumor antigen. J Virol. 1988 Apr;62(4):1258–1265. doi: 10.1128/jvi.62.4.1258-1265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson R., Cohen P., Lane D. P. Simian virus 40 large T-antigen-dependent DNA replication is activated by protein phosphatase 2A in vitro. J Virol. 1990 May;64(5):2380–2383. doi: 10.1128/jvi.64.5.2380-2383.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Momand J. Tumor suppressor genes: the p53 and retinoblastoma sensitivity genes and gene products. Biochim Biophys Acta. 1990 Jun 1;1032(1):119–136. doi: 10.1016/0304-419x(90)90015-s. [DOI] [PubMed] [Google Scholar]
- Levine A. J. The p53 protein and its interactions with the oncogene products of the small DNA tumor viruses. Virology. 1990 Aug;177(2):419–426. doi: 10.1016/0042-6822(90)90505-l. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Bradley M. K. The simian virus 40 large T antigen. A lot packed into a little. Mol Biol Med. 1987 Apr;4(2):63–80. [PubMed] [Google Scholar]
- Loeber G., Tevethia M. J., Schwedes J. F., Tegtmeyer P. Temperature-sensitive mutants identify crucial structural regions of simian virus 40 large T antigen. J Virol. 1989 Oct;63(10):4426–4430. doi: 10.1128/jvi.63.10.4426-4430.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey D., Brizuela L., Mohr I., Marshak D. R., Gluzman Y., Beach D. Phosphorylation of large tumour antigen by cdc2 stimulates SV40 DNA replication. Nature. 1989 Oct 12;341(6242):503–507. doi: 10.1038/341503a0. [DOI] [PubMed] [Google Scholar]
- Mohr I. J., Gluzman Y., Fairman M. P., Strauss M., McVey D., Stillman B., Gerard R. D. Production of simian virus 40 large tumor antigen in bacteria: altered DNA-binding specificity and dna-replication activity of underphosphorylated large tumor antigen. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6479–6483. doi: 10.1073/pnas.86.17.6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr I. J., Stillman B., Gluzman Y. Regulation of SV40 DNA replication by phosphorylation of T antigen. EMBO J. 1987 Jan;6(1):153–160. doi: 10.1002/j.1460-2075.1987.tb04733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan C. A., Brugge J. S., Butel J. S. Characterization of simian cells tranformed by temperature-sensitive mutants of simian virus 40. J Virol. 1976 Jun;18(3):1106–1119. doi: 10.1128/jvi.18.3.1106-1119.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patschinsky T., Bister K. Structural analysis of normal and transforming mil(raf) proteins: effect of 5'-truncation on phosphorylation in vivo or in vitro. Oncogene. 1988 Oct;3(4):357–364. [PubMed] [Google Scholar]
- Patschinsky T., Deppert W. Phosphorylation of p53 in primary, immortalised and transformed Balb/c mouse cells. Oncogene. 1990 Jul;5(7):1071–1076. [PubMed] [Google Scholar]
- Pintel D., Bouck N., di Mayorca G. Separation of lytic and transforming functions of the simian virus 40 A region: two mutants which are temperature sensitive for lytic functions have opposite effects on transformation. J Virol. 1981 May;38(2):518–528. doi: 10.1128/jvi.38.2.518-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollwein P., Wagner S., Knippers R. Application of an immunoprecipitation procedure to the study of SV40 tumor antigen interaction with mouse genomic DNA sequences. Nucleic Acids Res. 1987 Dec 10;15(23):9741–9759. doi: 10.1093/nar/15.23.9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C. The replication functions of SV40 T antigen are regulated by phosphorylation. Cell. 1990 Jun 1;61(5):735–738. doi: 10.1016/0092-8674(90)90179-i. [DOI] [PubMed] [Google Scholar]
- Richter W., Deppert W. The cellular chromatin is an important target for SV40 large T antigen in maintaining the transformed phenotype. Virology. 1990 Feb;174(2):543–556. doi: 10.1016/0042-6822(90)90108-4. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., La Thangue N. B., Murphy D., Skene B. I. The regulation of cellular transcription by Simian virus 40 large T-antigen. Proc R Soc Lond B Biol Sci. 1985 Oct 22;226(1242):15–23. doi: 10.1098/rspb.1985.0076. [DOI] [PubMed] [Google Scholar]
- Ryan K. W., Christensen J. B., Imperiale M. J., Brockman W. W. Isolation of a simian virus 40 T-antigen-positive, transformation-resistant cell line by indirect selection. Mol Cell Biol. 1985 Dec;5(12):3577–3582. doi: 10.1128/mcb.5.12.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Buck M., Schneider J., Kalderon D., Fanning E., Smith A. E. Biochemical characterization of phosphorylation site mutants of simian virus 40 large T antigen: evidence for interaction between amino- and carboxy-terminal domains. J Virol. 1991 Mar;65(3):1479–1490. doi: 10.1128/jvi.65.3.1479-1490.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Echle B., Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J Virol. 1982 Oct;44(1):116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Haber A. Simian virus 40 large T antigen induces or activates a protein kinase which phosphorylates the transformation-associated protein p53. J Virol. 1990 Feb;64(2):672–679. doi: 10.1128/jvi.64.2.672-679.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Hardung M., Echle B., Walter G. DNA-binding activity of simian virus 40 large T antigen correlates with a distinct phosphorylation state. J Virol. 1984 Apr;50(1):1–12. doi: 10.1128/jvi.50.1.1-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H. Phosphorylation of simian virus 40 large T antigen: cytoplasmic and nuclear phophorylation sites differ in their metabolic stability. Virology. 1986 Apr 15;150(1):85–95. [PubMed] [Google Scholar]
- Scheidtmann K. H., Schickedanz J., Walter G., Lanford R. E., Butel J. S. Differential phosphorylation of cytoplasmic and nuclear variants of simian virus 40 large T antigen encoded by simian virus 40-adenovirus 7 hybrid viruses. J Virol. 1984 May;50(2):636–640. doi: 10.1128/jvi.50.2.636-640.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmbeck R., Deppert W. Nuclear subcompartmentalization of simian virus 40 large T antigen: evidence for in vivo regulation of biochemical activities. J Virol. 1989 May;63(5):2308–2316. doi: 10.1128/jvi.63.5.2308-2316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Fanning E. Mutations in the phosphorylation sites of simian virus 40 (SV40) T antigen alter its origin DNA-binding specificity for sites I or II and affect SV40 DNA replication activity. J Virol. 1988 May;62(5):1598–1605. doi: 10.1128/jvi.62.5.1598-1605.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Knippers R. The simian virus 40 large tumor antigen. Biochim Biophys Acta. 1987 Oct 9;910(1):1–10. doi: 10.1016/0167-4781(87)90088-1. [DOI] [PubMed] [Google Scholar]
- Staufenbiel M., Deppert W. Different structural systems of the nucleus are targets for SV40 large T antigen. Cell. 1983 May;33(1):173–181. doi: 10.1016/0092-8674(83)90346-x. [DOI] [PubMed] [Google Scholar]
- Staufenbiel M., Deppert W. Preparation of nuclear matrices from cultured cells: subfractionation of nuclei in situ. J Cell Biol. 1984 May;98(5):1886–1894. doi: 10.1083/jcb.98.5.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmeyer K., Deppert W. DNA binding properties of murine p53. Oncogene. 1988 Nov;3(5):501–507. [PubMed] [Google Scholar]
- Van Roy F., Fransen L., Fiers W. Metabolic turnover of phosphorylation sites in simian virus 40 large T antigen. J Virol. 1983 Jan;45(1):442–446. doi: 10.1128/jvi.45.1.442-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup D. M., Kauffman M. G., Kelly T. J. Activation of SV40 DNA replication in vitro by cellular protein phosphatase 2A. EMBO J. 1989 Dec 1;8(12):3891–3898. doi: 10.1002/j.1460-2075.1989.tb08568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B., Vakalopoulou E., Fanning E. Allosteric control of simian virus 40 T-antigen binding to viral origin DNA. J Virol. 1986 Jun;58(3):765–772. doi: 10.1128/jvi.58.3.765-772.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S., Knippers R. An SV40 large T antigen binding site in the cellular genome is part of a cis-acting transcriptional element. Oncogene. 1990 Mar;5(3):353–359. [PubMed] [Google Scholar]
- White R. J., Stott D., Rigby P. W. Regulation of RNA polymerase III transcription in response to Simian virus 40 transformation. EMBO J. 1990 Nov;9(11):3713–3721. doi: 10.1002/j.1460-2075.1990.tb07584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roy F., Fransen L., Fiers W. Improved localization of phosphorylation sites in simian virus 40 large T antigen. J Virol. 1983 Jan;45(1):315–331. doi: 10.1128/jvi.45.1.315-331.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]