Abstract
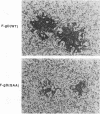
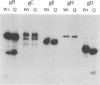
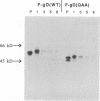
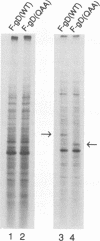
Glycoprotein D (gD) is an envelope component of herpes simplex virus essential for virus penetration. gD contains three sites for addition of asparagine-linked carbohydrates (N-CHO), all of which are utilized. Previously, we characterized mutant forms of herpes simplex virus type 1 gD (gD-1) lacking one or all three N-CHO addition sites. All of the mutants complemented the infectivity of a gD-minus virus, F-gD beta, to the same extent as wild-type gD. Here, we show that recombinant viruses containing mutations in the gD-1 gene which eliminate the three N-CHO signals are viable. Two such viruses, called F-gD(QAA)-1 and F-gD(QAA)-2, were independently isolated, and the three mutations in the gD gene in one of these viruses were verified by DNA sequencing. We also verified that the gD produced in cells infected by these viruses is devoid of N-CHO. Plaques formed by both mutants developed more slowly than those of the wild-type control virus, F-gD(WT), and were approximately one-half the size of the wild-type. One mutant, F-gD(QAA)-2, was selected for further study. The QAA mutant and wild-type gD proteins extracted from infected cells differed in structure, as determined by the binding of monoclonal antibodies to discontinuous epitopes. However, flow cytometry analysis showed that the amount and structure of gD found on infected cell surfaces was unaffected by the presence or absence of N-CHO. Other properties of F-gD(QAA)-2 were quite similar to those of F-gD(WT). These included (i) the kinetics of virus production as well as the intracellular and extracellular virus titers; (ii) the rate of virus entry into uninfected cells; (iii) the levels of gB, gC, gE, gH, and gI expressed by infected cells; and (iv) the turnover time of gD. Thus, the absence of N-CHO from gD-1 has some effect on its structure but very little effect on its function in virus infection in cell culture.
Full text
PDF


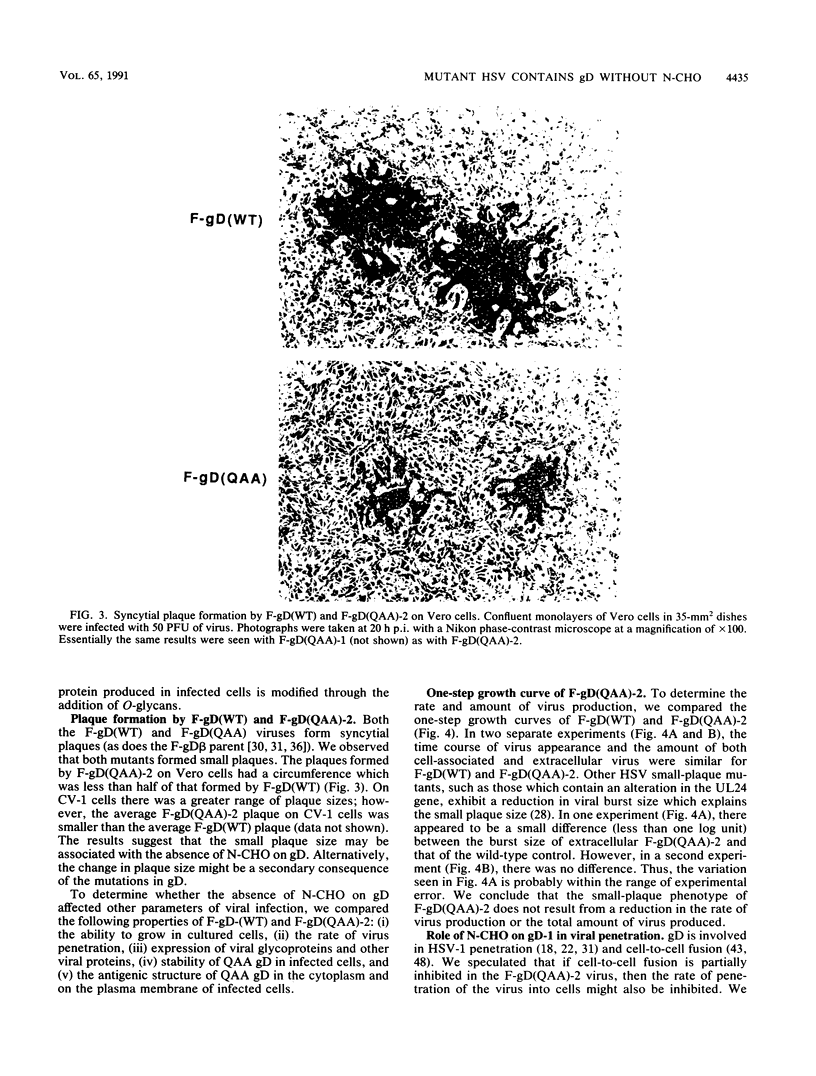
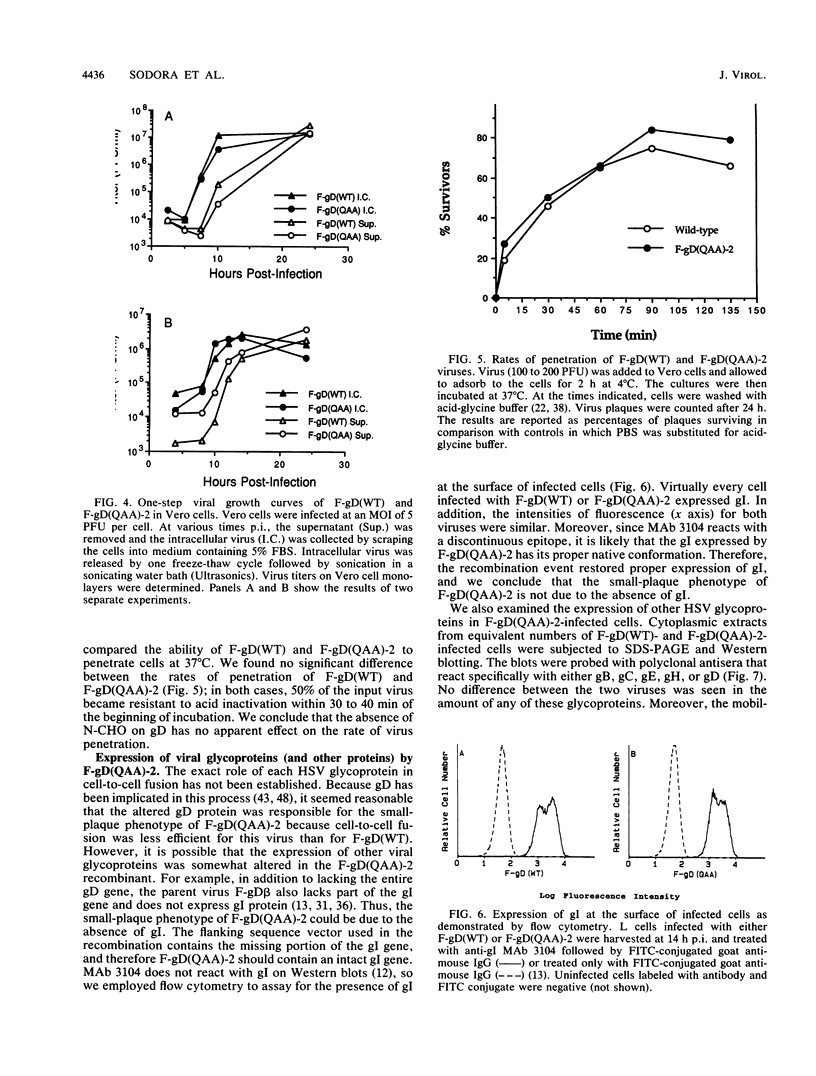

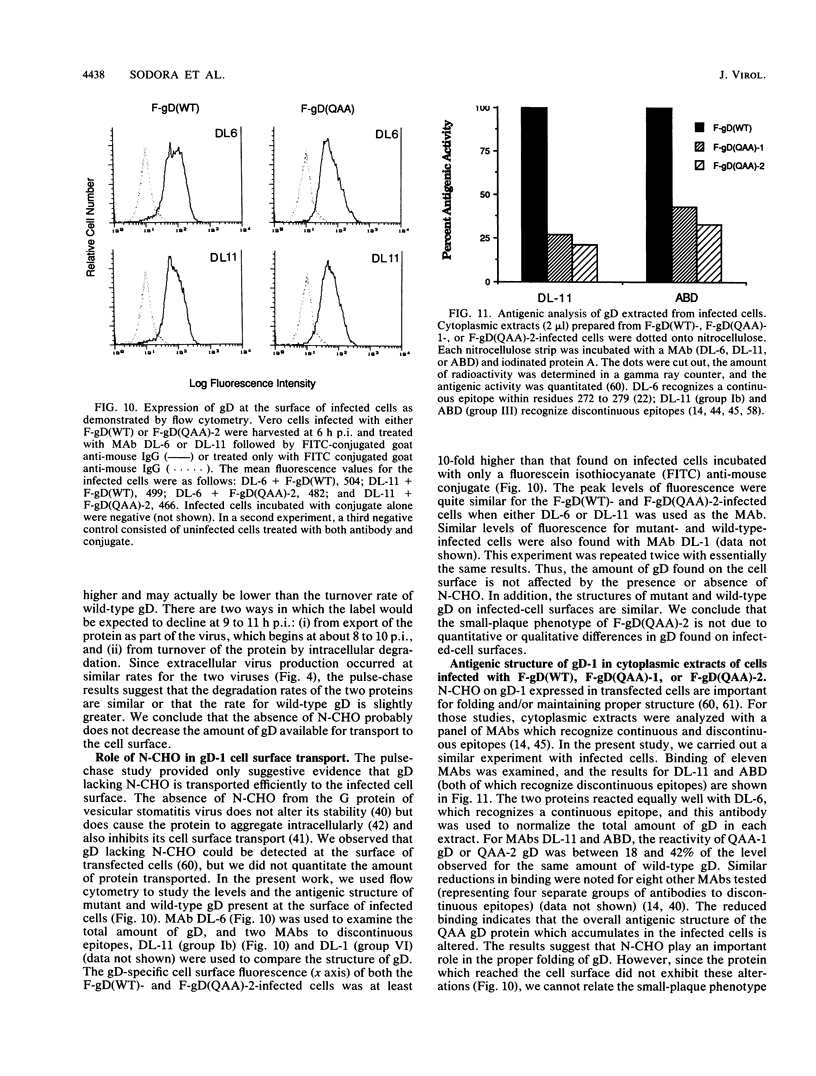



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cai W. H., Gu B., Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988 Aug;62(8):2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W. Z., Person S., Warner S. C., Zhou J. H., DeLuca N. A. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J Virol. 1987 Mar;61(3):714–721. doi: 10.1128/jvi.61.3.714-721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Poletti L., Dall'Olio F., Serafini-Cessi F. Infectivity and glycoprotein processing of herpes simplex virus type 1 grown in a ricin-resistant cell line deficient in N-acetylglucosaminyl transferase I. J Virol. 1982 Sep;43(3):1061–1071. doi: 10.1128/jvi.43.3.1061-1071.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Qi S., Avitabile E., Foà-Tomasi L., Brandimarti R., Roizman B. Glycoprotein D of herpes simplex virus encodes a domain which precludes penetration of cells expressing the glycoprotein by superinfecting herpes simplex virus. J Virol. 1990 Dec;64(12):6070–6079. doi: 10.1128/jvi.64.12.6070-6079.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F. K., Trimble R. B., Maley F. The effect of carbohydrate depletion on the properties of yeast external invertase. J Biol Chem. 1978 Dec 25;253(24):8691–8693. [PubMed] [Google Scholar]
- Cohen G. H., Dietzschold B., Ponce de Leon M., Long D., Golub E., Varrichio A., Pereira L., Eisenberg R. J. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984 Jan;49(1):102–108. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Wilcox W. C., Sodora D. L., Long D., Levin J. Z., Eisenberg R. J. Expression of herpes simplex virus type 1 glycoprotein D deletion mutants in mammalian cells. J Virol. 1988 Jun;62(6):1932–1940. doi: 10.1128/jvi.62.6.1932-1940.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P. J., Schaffer P. A., Minson A. C. Excretion of non-infectious virus particles lacking glycoprotein H by a temperature-sensitive mutant of herpes simplex virus type 1: evidence that gH is essential for virion infectivity. J Gen Virol. 1988 Jun;69(Pt 6):1147–1156. doi: 10.1099/0022-1317-69-6-1147. [DOI] [PubMed] [Google Scholar]
- Dubin G., Frank I., Friedman H. M. Herpes simplex virus type 1 encodes two Fc receptors which have different binding characteristics for monomeric immunoglobulin G (IgG) and IgG complexes. J Virol. 1990 Jun;64(6):2725–2731. doi: 10.1128/jvi.64.6.2725-2731.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Friedman H. M., Fries L. F., Frank M. M., Hastings J. C., Cohen G. H. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb Pathog. 1987 Dec;3(6):423–435. doi: 10.1016/0882-4010(87)90012-x. [DOI] [PubMed] [Google Scholar]
- Flowers C. C., Eastman E. M., O'Callaghan D. J. Sequence analysis of a glycoprotein D gene homolog within the unique short segment of the EHV-1 genome. Virology. 1991 Jan;180(1):175–184. doi: 10.1016/0042-6822(91)90021-3. [DOI] [PubMed] [Google Scholar]
- Fuller A. O., Santos R. E., Spear P. G. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989 Aug;63(8):3435–3443. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher W. R., Levitan D. B., Blough H. A. Effect of 2-deoxy-D-glucose on cell fusion induced by Newcastle disease and herpes simplex viruses. Virology. 1973 Sep;55(1):193–201. doi: 10.1016/s0042-6822(73)81021-9. [DOI] [PubMed] [Google Scholar]
- HUANG A. S., WAGNER R. R. PENETRATION OF HERPES SIMPLEX VIRUS INTO HUMAN EPIDERMOID CELLS. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:863–869. doi: 10.3181/00379727-116-29392. [DOI] [PubMed] [Google Scholar]
- Heeg U., Dienes H. P., Müller S., Falke D. Involvement of actin-containing microfilaments in HSV-induced cytopathology and the influence of inhibitors of glycosylation. Arch Virol. 1986;91(3-4):257–270. doi: 10.1007/BF01314285. [DOI] [PubMed] [Google Scholar]
- Herold B. C., WuDunn D., Soltys N., Spear P. G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991 Mar;65(3):1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlander S. L., Sutherland S. L., Gage P. J., Johnson D. C., Levine M., Glorioso J. C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987 Nov;61(11):3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Bole D. G., Hoover-Litty H., Helenius A., Copeland C. S. Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP). J Cell Biol. 1989 Jun;108(6):2117–2126. doi: 10.1083/jcb.108.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Isola V. J., Eisenberg R. J., Siebert G. R., Heilman C. J., Wilcox W. C., Cohen G. H. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J Virol. 1989 May;63(5):2325–2334. doi: 10.1128/jvi.63.5.2325-2334.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi K. M., Stevens J. G. Molecular and biological characterization of a herpes simplex virus type 1 (HSV-1) neuroinvasiveness gene. J Exp Med. 1990 Aug 1;172(2):487–496. doi: 10.1084/jem.172.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson J. G., Martin S. L., Coen D. M. A conserved open reading frame that overlaps the herpes simplex virus thymidine kinase gene is important for viral growth in cell culture. J Virol. 1989 Apr;63(4):1839–1843. doi: 10.1128/jvi.63.4.1839-1843.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990 Aug;15(8):291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Ligas M. W. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J Virol. 1988 Dec;62(12):4605–4612. doi: 10.1128/jvi.62.12.4605-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Wittels M., Spear P. G. Binding to cells of virosomes containing herpes simplex virus type 1 glycoproteins and evidence for fusion. J Virol. 1984 Oct;52(1):238–247. doi: 10.1128/jvi.52.1.238-247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky K. F., Call S. M., Dower S. K., Krieger M. Abnormal intracellular sorting of O-linked carbohydrate-deficient interleukin-2 receptors. Mol Cell Biol. 1988 Aug;8(8):3357–3363. doi: 10.1128/mcb.8.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn J. E., Eing B. R., Brossmer R., Munk K., Braun R. W. Removal of N-linked carbohydrates decreases the infectivity of herpes simplex virus type 1. J Gen Virol. 1988 Nov;69(Pt 11):2847–2858. doi: 10.1099/0022-1317-69-11-2847. [DOI] [PubMed] [Google Scholar]
- Ligas M. W., Johnson D. C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988 May;62(5):1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D., Cohen G. H., Muggeridge M. I., Eisenberg R. J. Cysteine mutants of herpes simplex virus type 1 glycoprotein D exhibit temperature-sensitive properties in structure and function. J Virol. 1990 Nov;64(11):5542–5552. doi: 10.1128/jvi.64.11.5542-5552.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Doms R. W., Bole D. G., Helenius A., Rose J. K. Heavy chain binding protein recognizes incompletely disulfide-bonded forms of vesicular stomatitis virus G protein. J Biol Chem. 1990 Apr 25;265(12):6879–6883. [PubMed] [Google Scholar]
- Machamer C. E., Florkiewicz R. Z., Rose J. K. A single N-linked oligosaccharide at either of the two normal sites is sufficient for transport of vesicular stomatitis virus G protein to the cell surface. Mol Cell Biol. 1985 Nov;5(11):3074–3083. doi: 10.1128/mcb.5.11.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. Influence of new glycosylation sites on expression of the vesicular stomatitis virus G protein at the plasma membrane. J Biol Chem. 1988 Apr 25;263(12):5948–5954. [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. Vesicular stomatitis virus G proteins with altered glycosylation sites display temperature-sensitive intracellular transport and are subject to aberrant intermolecular disulfide bonding. J Biol Chem. 1988 Apr 25;263(12):5955–5960. [PubMed] [Google Scholar]
- Minson A. C., Hodgman T. C., Digard P., Hancock D. C., Bell S. E., Buckmaster E. A. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J Gen Virol. 1986 Jun;67(Pt 6):1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- Muggeridge M. I., Isola V. J., Byrn R. A., Tucker T. J., Minson A. C., Glorioso J. C., Cohen G. H., Eisenberg R. J. Antigenic analysis of a major neutralization site of herpes simplex virus glycoprotein D, using deletion mutants and monoclonal antibody-resistant mutants. J Virol. 1988 Sep;62(9):3274–3280. doi: 10.1128/jvi.62.9.3274-3280.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggeridge M. I., Wu T. T., Johnson D. C., Glorioso J. C., Eisenberg R. J., Cohen G. H. Antigenic and functional analysis of a neutralization site of HSV-1 glycoprotein D. Virology. 1990 Feb;174(2):375–387. doi: 10.1016/0042-6822(90)90091-5. [DOI] [PubMed] [Google Scholar]
- Ng D. T., Hiebert S. W., Lamb R. A. Different roles of individual N-linked oligosaccharide chains in folding, assembly, and transport of the simian virus 5 hemagglutinin-neuraminidase. Mol Cell Biol. 1990 May;10(5):1989–2001. doi: 10.1128/mcb.10.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble A. G., Lee G. T., Sprague R., Parish M. L., Spear P. G. Anti-gD monoclonal antibodies inhibit cell fusion induced by herpes simplex virus type 1. Virology. 1983 Aug;129(1):218–224. doi: 10.1016/0042-6822(83)90409-9. [DOI] [PubMed] [Google Scholar]
- PLUMMER T. H., Jr, HIRS C. H. ON THE STRUCTURE OF BOVINE PANCREATIC RIBONUCLEASE B. ISOLATION OF A GLYCOPEPTIDE. J Biol Chem. 1964 Aug;239:2530–2538. [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Armentrout M. A., Marchioli C. C., Yancey R. J., Jr, Post L. E. DNA sequence of the gene for pseudorabies virus gp50, a glycoprotein without N-linked glycosylation. J Virol. 1986 Aug;59(2):216–223. doi: 10.1128/jvi.59.2.216-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneurin J. M., Desgranges C., Seigneurin D., Paire J., Renversez J. C., Jacquemont B., Micouin C. Herpes simplex virus glycoprotein D: human monoclonal antibody produced by bone marrow cell line. Science. 1983 Jul 8;221(4606):173–175. doi: 10.1126/science.6304881. [DOI] [PubMed] [Google Scholar]
- Serafini-Cessi F., Dall'Olio F., Malagolini N., Pereira L., Campadelli-Fiume G. Comparative study on O-linked oligosaccharides of glycoprotein D of herpes simplex virus types 1 and 2. J Gen Virol. 1988 Apr;69(Pt 4):869–877. doi: 10.1099/0022-1317-69-4-869. [DOI] [PubMed] [Google Scholar]
- Sodora D. L., Cohen G. H., Eisenberg R. J. Influence of asparagine-linked oligosaccharides on antigenicity, processing, and cell surface expression of herpes simplex virus type 1 glycoprotein D. J Virol. 1989 Dec;63(12):5184–5193. doi: 10.1128/jvi.63.12.5184-5193.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodora D. L., Cohen G. H., Muggeridge M. I., Eisenberg R. J. Absence of asparagine-linked oligosaccharides from glycoprotein D of herpes simplex virus type 1 results in a structurally altered but biologically active protein. J Virol. 1991 Aug;65(8):4424–4431. doi: 10.1128/jvi.65.8.4424-4431.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Prusoff W. H., Tritton T. R. A study of the antiviral mechanism of action of 2-deoxy-D-glucose: normally glycosylated proteins are not strictly required for herpes simplex virus attachment but increase viral penetration and infectivity. Virology. 1982 Nov;123(1):123–138. doi: 10.1016/0042-6822(82)90300-2. [DOI] [PubMed] [Google Scholar]
- Svennerholm B., Olofsson S., Lundén R., Vahlne A., Lycke E. Adsorption and penetration of enveloped herpes simplex virus particles modified by tunicamycin or 2-deoxy-D-glucose. J Gen Virol. 1982 Dec;63(2):343–349. doi: 10.1099/0022-1317-63-2-343. [DOI] [PubMed] [Google Scholar]
- Taylor A. K., Wall R. Selective removal of alpha heavy-chain glycosylation sites causes immunoglobulin A degradation and reduced secretion. Mol Cell Biol. 1988 Oct;8(10):4197–4203. doi: 10.1128/mcb.8.10.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikoo S. K., Fitzpatrick D. R., Babiuk L. A., Zamb T. J. Molecular cloning, sequencing, and expression of functional bovine herpesvirus 1 glycoprotein gIV in transfected bovine cells. J Virol. 1990 Oct;64(10):5132–5142. doi: 10.1128/jvi.64.10.5132-5142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox W. C., Long D., Sodora D. L., Eisenberg R. J., Cohen G. H. The contribution of cysteine residues to antigenicity and extent of processing of herpes simplex virus type 1 glycoprotein D. J Virol. 1988 Jun;62(6):1941–1947. doi: 10.1128/jvi.62.6.1941-1947.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittels M., Spear P. G. Penetration of cells by herpes simplex virus does not require a low pH-dependent endocytic pathway. Virus Res. 1991 Mar;18(2-3):271–290. doi: 10.1016/0168-1702(91)90024-p. [DOI] [PubMed] [Google Scholar]
- Zhu B. C., Fisher S. F., Pande H., Calaycay J., Shively J. E., Laine R. A. Human placental (fetal) fibronectin: increased glycosylation and higher protease resistance than plasma fibronectin. Presence of polylactosamine glycopeptides and properties of a 44-kilodalton chymotryptic collagen-binding domain: difference from human plasma fibronectin. J Biol Chem. 1984 Mar 25;259(6):3962–3970. [PubMed] [Google Scholar]