Abstract
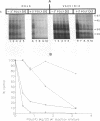
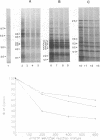
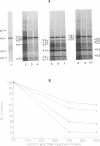
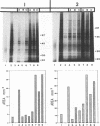
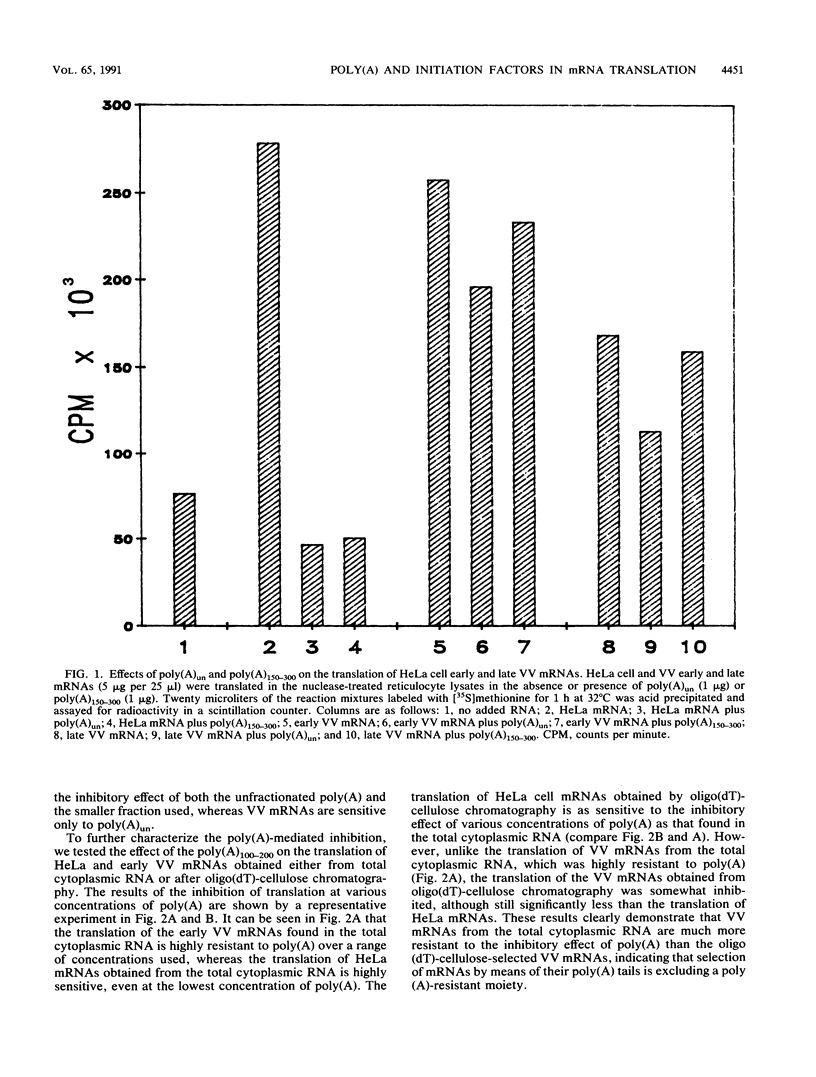
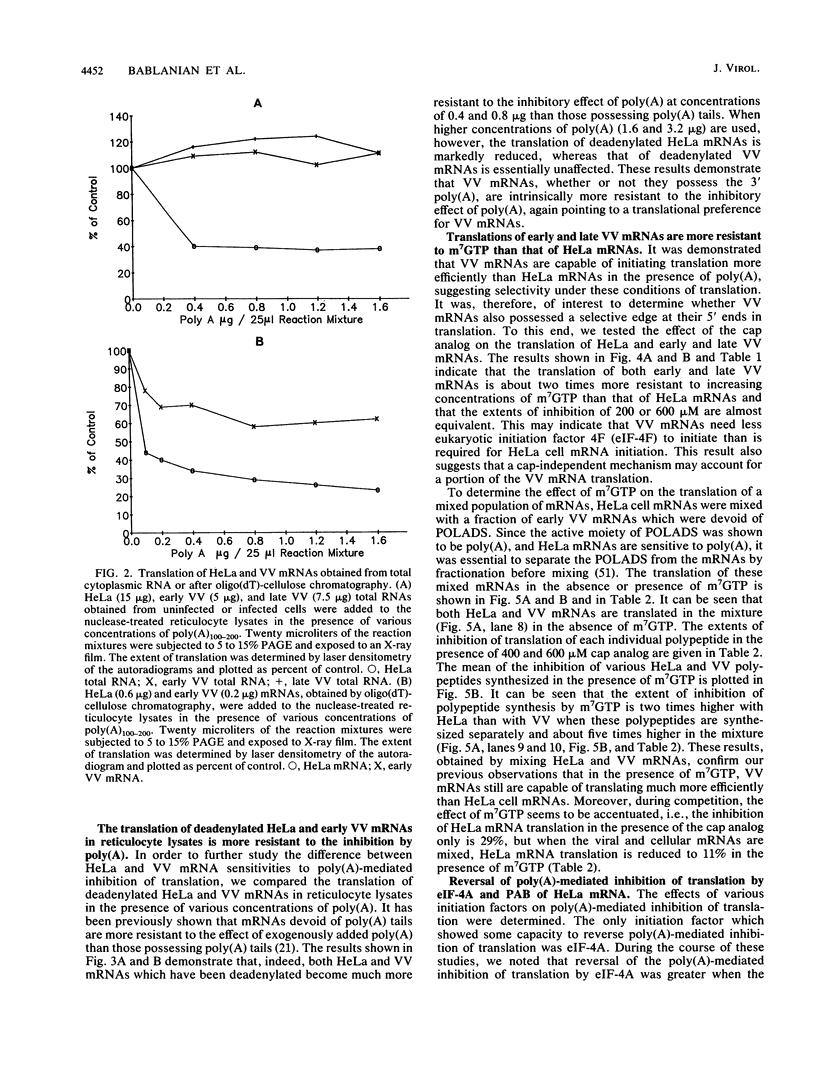
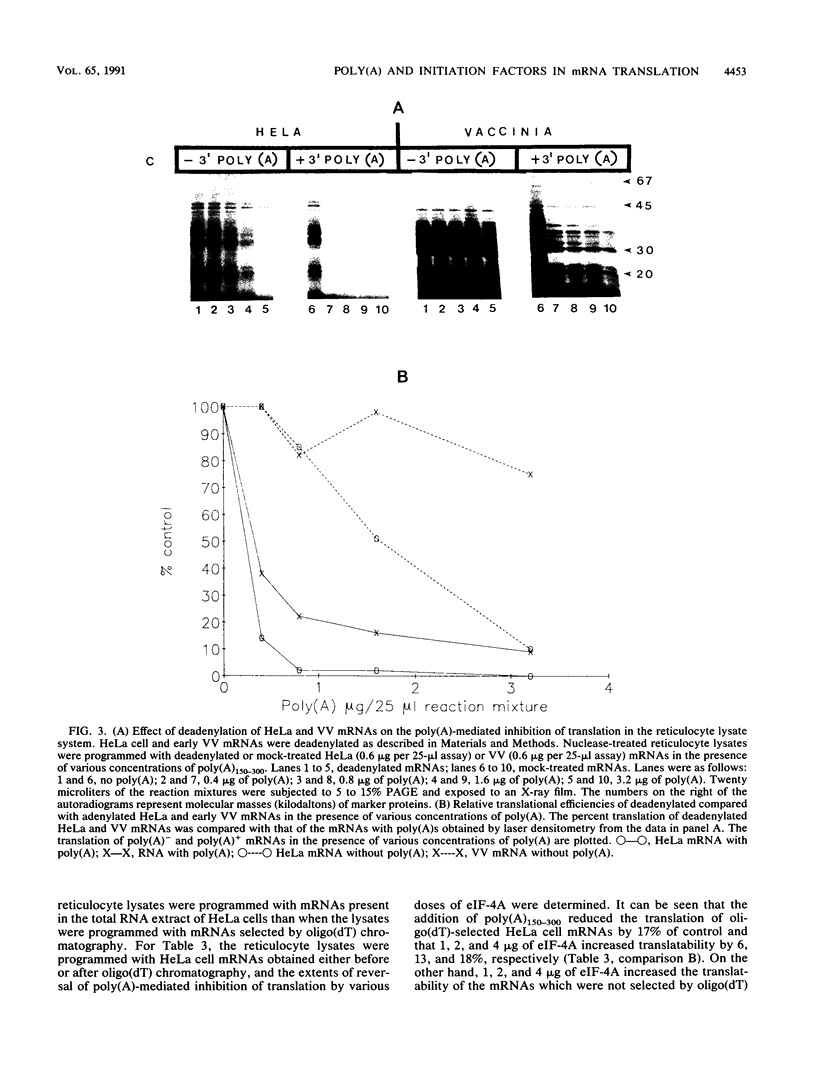
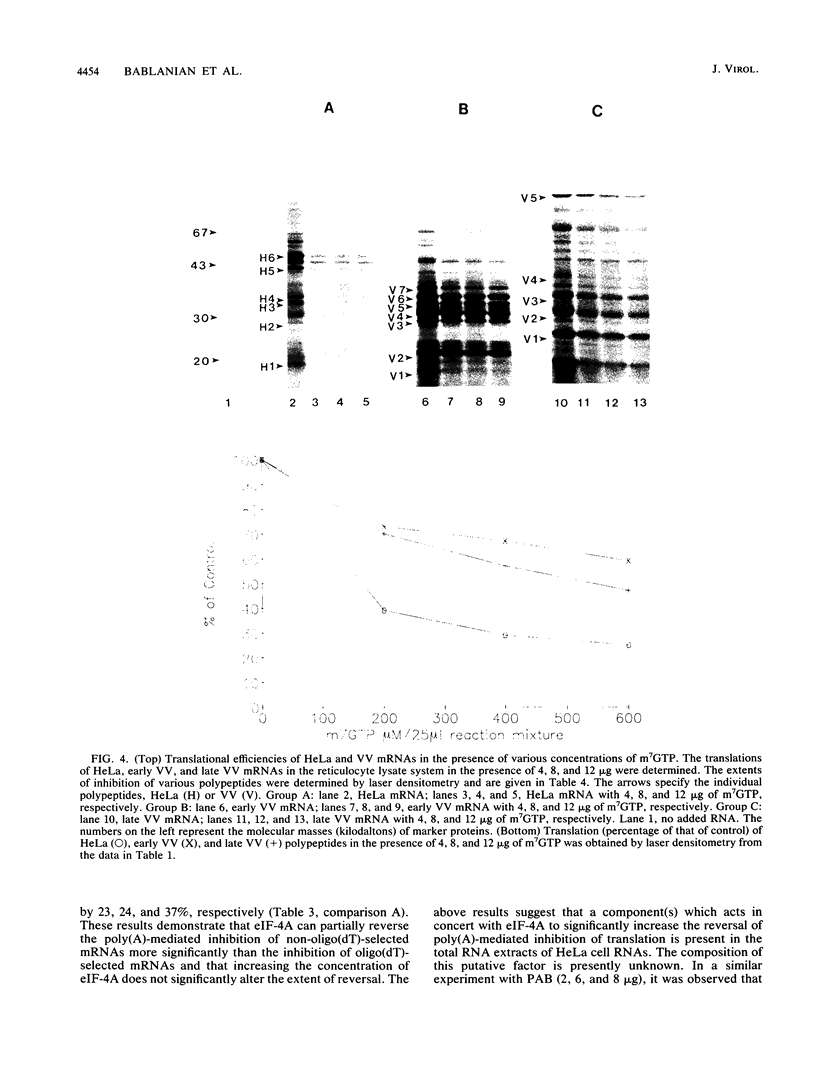
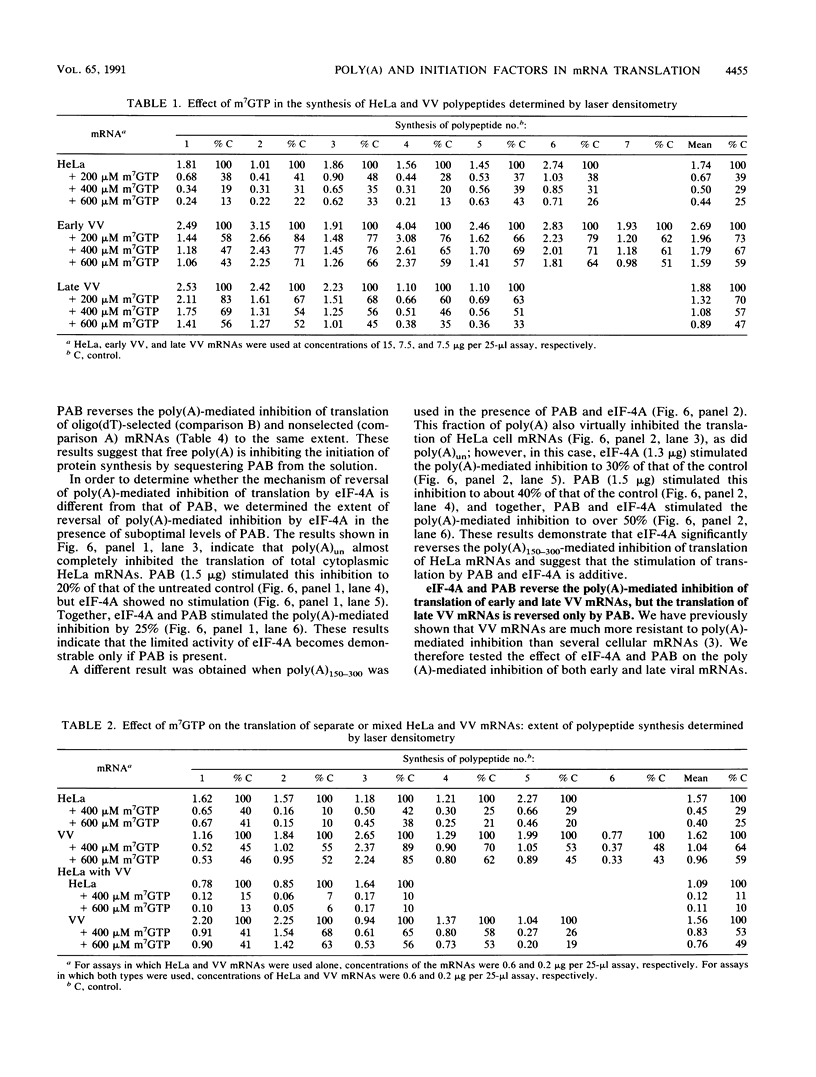
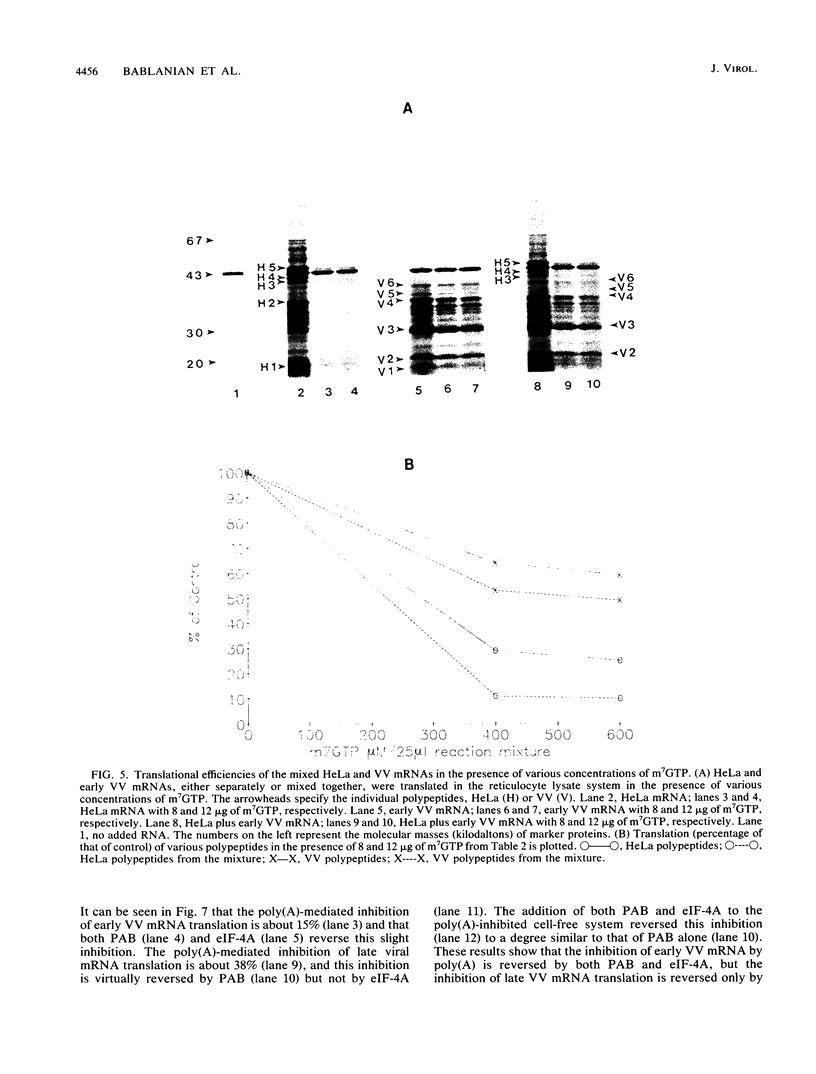
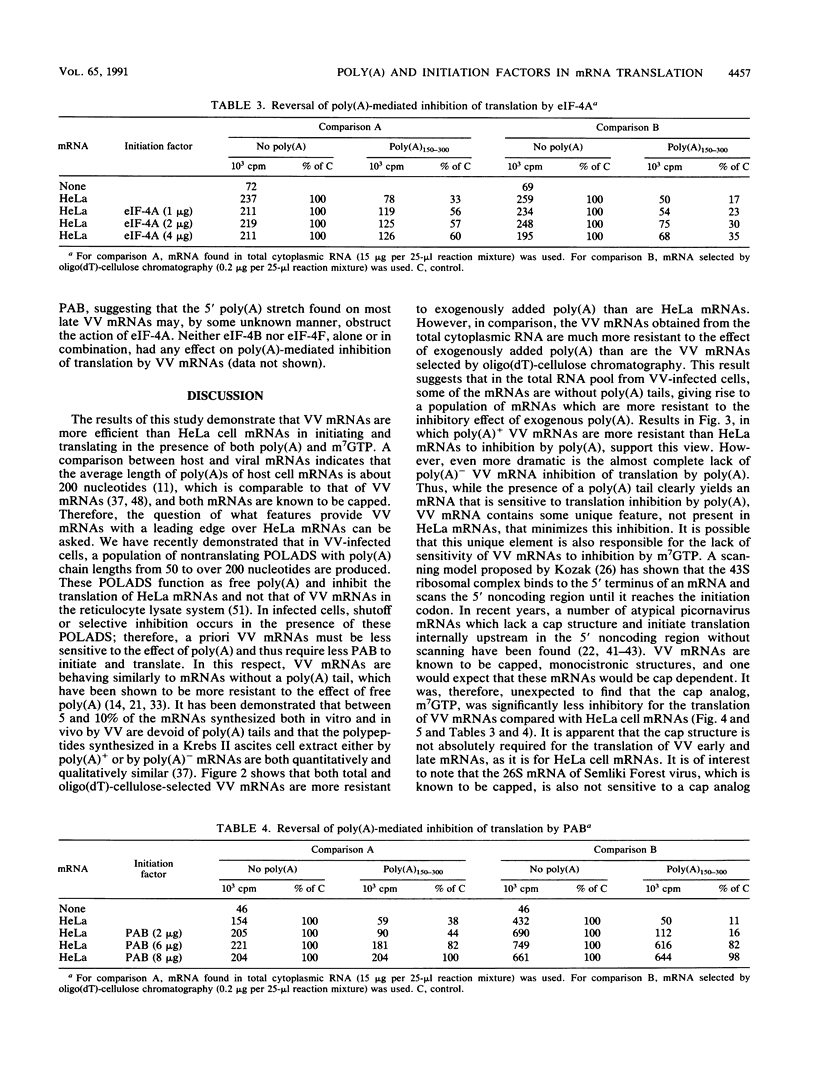
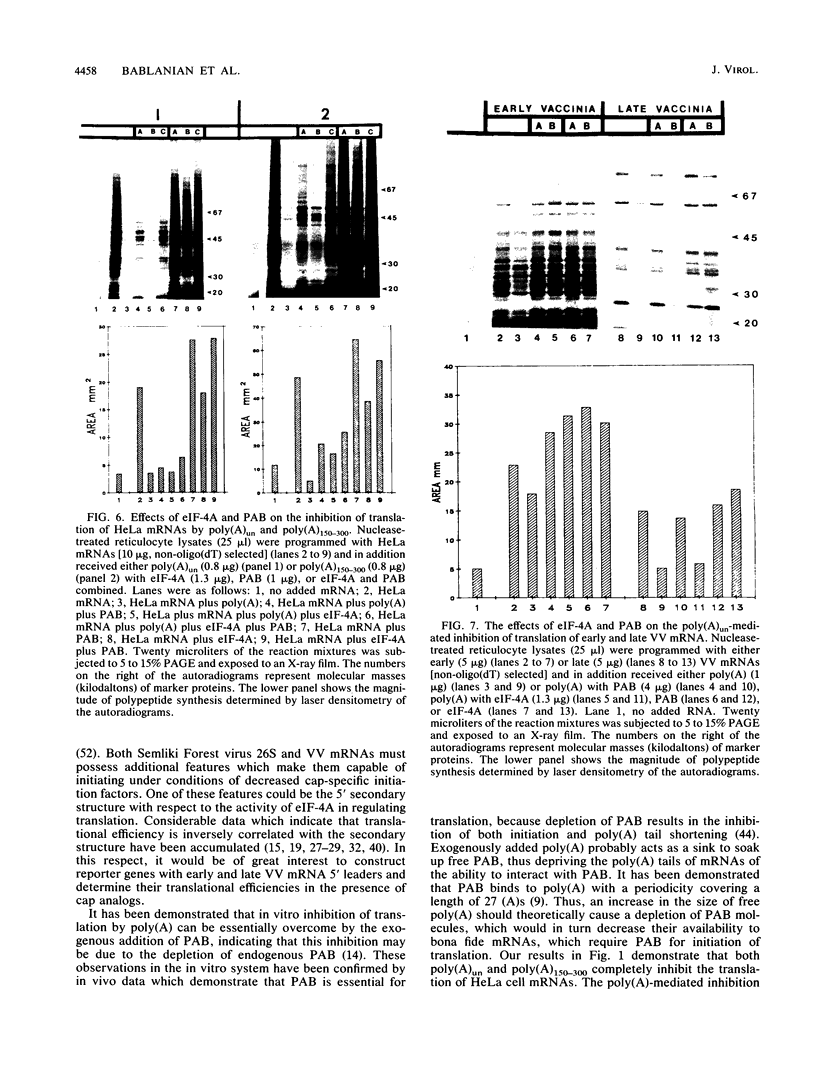
We have recently demonstrated that the poly(A) moieties of short RNAs obtained from both in vitro transcription and from vaccinia virus (VV)-infected cells exhibit dissimilar effects on the in vitro translation of cellular and VV mRNAs (R. Bablanian, G. Coppola, P. Masters, and A. K. Banerjee, Virology 148:375-380, 1986; M. J. Su and R. Bablanian, Virology 179:679-693, 1990). In the present study, we have investigated the roles of poly(A), m7GTP, and initiation factors in the mechanism of selective translation of VV mRNAs. The effects of unfractionated poly(A) [termed poly(A)un, with various chain lengths up to 3,000 nucleotides] and a 150- to 300-nucleotide fraction of synthetic poly(A) [termed poly(A)150-300] on the translation of HeLa cell mRNAs and early and late VV mRNAs were studied. Both the poly(A)un and the poly(A)150-300 completely inhibited the translation of HeLa cell mRNAs obtained from total cytoplasmic RNA in the nuclease-treated reticulocyte lysates. Viral mRNAs from total cytoplasmic RNA also were slightly inhibited (15 to 38%) by the poly(A)un, whereas the poly(A)150-300 had no significant effect on their translation. The translation of oligo(dT)-cellulose-selected HeLa mRNAs was as sensitive to inhibition by poly(A)150-300 as the mRNAs found in total cytoplasmic RNA. However, the translations of oligo(dT)-cellulose-selected viral mRNAs become more sensitive to the inhibitory effect of poly(A)150-300 than the translations of viral mRNAs found in the total cytoplasmic RNA. Both HeLa and VV mRNAs became more resistant to the poly(A)-mediated inhibition when these mRNAs were deadenylated, but the relative resistance to inhibition by poly(A)150-300 of deadenylated VV mRNAs was much greater than that of HeLa cell mRNAs. The translation of VV mRNAs was significantly less inhibited than the translation of HeLa mRNAs when the cap analog, m7GTP, was added to the cell-free system. The inhibition of HeLa cell mRNA translation by both poly(A)un and poly(A)150-300 was completely restored when poly(A)-binding protein (PAB) was added to the cell-free translational system. The addition of eukaryotic initiation factor 4A (eIF-4A) did not restore translation when poly(A)un was used to inhibit translation; however, inhibition by poly(A)150-300 was significantly reversed by this initiation factor. The reversal of poly (A)-mediated inhibition of HeLa cell mRNA translation was additive when PAB was used together with eIF-4A. Early VV mRNA translation was only slightly inhibited by poly(A)un (15%), and this inhibition was completely reversed by either PAB or eIF-4A.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn B. Y., Moss B. Capped poly(A) leaders of variable lengths at the 5' ends of vaccinia virus late mRNAs. J Virol. 1989 Jan;63(1):226–232. doi: 10.1128/jvi.63.1.226-232.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bablanian R., Banerjee A. K. Poly(riboadenylic acid) preferentially inhibits in vitro translation of cellular mRNAs compared with vaccinia virus mRNAs: possible role in vaccinia virus cytopathology. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1290–1294. doi: 10.1073/pnas.83.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bablanian R., Coppola G., Masters P. S., Banerjee A. K. Characterization of vaccinia virus transcripts involved in selective inhibition of host protein synthesis. Virology. 1986 Jan 30;148(2):375–380. doi: 10.1016/0042-6822(86)90334-x. [DOI] [PubMed] [Google Scholar]
- Bablanian R., Coppola G., Scribani S., Esteban M. Inhibition of protein synthesis by vaccinia virus. III. The effect of ultraviolet-irradiated virus on the inhibition of protein synthesis. Virology. 1981 Jul 15;112(1):1–12. doi: 10.1016/0042-6822(81)90606-1. [DOI] [PubMed] [Google Scholar]
- Bablanian R., Coppola G., Scribani S., Esteban M. Inhibition of protein synthesis by vaccinia virus. IV. The role of low-molecular-weight viral RNA in the inhibition of protein synthesis. Virology. 1981 Jul 15;112(1):13–24. doi: 10.1016/0042-6822(81)90607-3. [DOI] [PubMed] [Google Scholar]
- Bablanian R., Esteban M., Baxt B., Sonnabend J. A. Studies on the mechanisms of vaccina virus cytopathic effects. I. Inhibition of protein synthesis in infected cells is associated with virus-induced RNA synthesis. J Gen Virol. 1978 Jun;39(3):391–402. doi: 10.1099/0022-1317-39-3-391. [DOI] [PubMed] [Google Scholar]
- Bablanian R., Goswami S. K., Esteban M., Banerjee A. K. Selective inhibition of protein synthesis by synthetic and vaccinia virus-core synthesized poly(riboadenylic acids). Virology. 1987 Dec;161(2):366–373. doi: 10.1016/0042-6822(87)90129-2. [DOI] [PubMed] [Google Scholar]
- Baer B. W., Kornberg R. D. Repeating structure of cytoplasmic poly(A)-ribonucleoprotein. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1890–1892. doi: 10.1073/pnas.77.4.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanian R. Structural and functional alterations in cultured cells infected with cytocidal viruses. Prog Med Virol. 1975;19:40–83. [PubMed] [Google Scholar]
- Bertholet C., Van Meir E., ten Heggeler-Bordier B., Wittek R. Vaccinia virus produces late mRNAs by discontinuous synthesis. Cell. 1987 Jul 17;50(2):153–162. doi: 10.1016/0092-8674(87)90211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. The Role of the poly(A) sequence in mammalian messenger RNA. CRC Crit Rev Biochem. 1981;10(1):1–38. doi: 10.3109/10409238109114634. [DOI] [PubMed] [Google Scholar]
- Brown M., Dorson J. W., Bollum F. J. Terminal riboadenylate transferase: a poly A polymerase in purified vaccinia virus. J Virol. 1973 Aug;12(2):203–208. doi: 10.1128/jvi.12.2.203-208.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G., Bablanian R. Discriminatory inhibition of protein synthesis in cell-free systems by vaccinia virus transcripts. Proc Natl Acad Sci U S A. 1983 Jan;80(1):75–79. doi: 10.1073/pnas.80.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Leis J. P., Morgan M. A., Shatkin A. J., Merrick W. C. Characterization of eukaryotic initiation factor 4A, a protein involved in ATP-dependent binding of globin mRNA. J Biol Chem. 1982 May 10;257(9):5246–5252. [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Morgan M. A., Shatkin A. J., Merrick W. C. New initiation factor activity required for globin mRNA translation. J Biol Chem. 1983 May 10;258(9):5804–5810. [PubMed] [Google Scholar]
- Grossi de Sa M. F., Standart N., Martins de Sa C., Akhayat O., Huesca M., Scherrer K. The poly(A)-binding protein facilitates in vitro translation of poly(A)-rich mRNA. Eur J Biochem. 1988 Oct 1;176(3):521–526. doi: 10.1111/j.1432-1033.1988.tb14309.x. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Weiner H. Influence of the 5'-end region of aldehyde dehydrogenase mRNA on translational efficiency. Potential secondary structure inhibition of translation in vitro. J Biol Chem. 1989 Oct 25;264(30):17764–17769. [PubMed] [Google Scholar]
- Ink B. S., Pickup D. J. Vaccinia virus directs the synthesis of early mRNAs containing 5' poly(A) sequences. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1536–1540. doi: 10.1073/pnas.87.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Jacobson A., Favreau M. Possible involvement of poly(A) in protein synthesis. Nucleic Acids Res. 1983 Sep 24;11(18):6353–6368. doi: 10.1093/nar/11.18.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Kräusslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., Wimmer E. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988 Aug;62(8):2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. I. The mechanism of synthesis and release of RNA in vaccinia cores. J Mol Biol. 1970 May 28;50(1):1–18. doi: 10.1016/0022-2836(70)90100-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol Cell Biol. 1989 Nov;9(11):5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. Influence of mRNA secondary structure on binding and migration of 40S ribosomal subunits. Cell. 1980 Jan;19(1):79–90. doi: 10.1016/0092-8674(80)90390-6. [DOI] [PubMed] [Google Scholar]
- Kozak M. Leader length and secondary structure modulate mRNA function under conditions of stress. Mol Cell Biol. 1988 Jul;8(7):2737–2744. doi: 10.1128/mcb.8.7.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawson T. G., Ray B. K., Dodds J. T., Grifo J. A., Abramson R. D., Merrick W. C., Betsch D. F., Weith H. L., Thach R. E. Influence of 5' proximal secondary structure on the translational efficiency of eukaryotic mRNAs and on their interaction with initiation factors. J Biol Chem. 1986 Oct 25;261(30):13979–13989. [PubMed] [Google Scholar]
- Lemay G., Millward S. Inhibition of translation in L-cell lysates by free polyadenylic acid: differences in sensitivity among different mRNAs and possible involvement of an initiation factor. Arch Biochem Biophys. 1986 Aug 15;249(1):191–198. doi: 10.1016/0003-9861(86)90574-6. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Nathan D. G. Regulation of hemoglobin synthesis. Preferential inhibition of and globin synthesis. J Biol Chem. 1972 Dec 10;247(23):7822–7829. [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N. Vaccinia virus polyriboadenylate polymerase: convalent linkage of the product with polyribonucleotide and polydeoxyribonucleotide primers. J Virol. 1974 Jul;14(1):86–98. doi: 10.1128/jvi.14.1.86-98.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe D., Jacobson A. mRNA poly(A) tail, a 3' enhancer of translational initiation. Mol Cell Biol. 1990 Jul;10(7):3441–3455. doi: 10.1128/mcb.10.7.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Poly (A) sequences of vaccinia virus messenger RNA: nature, mode of addition and function during translation in vitra and in vivo. Virology. 1975 Jan;63(1):1–14. doi: 10.1016/0042-6822(75)90365-7. [DOI] [PubMed] [Google Scholar]
- Patel D. D., Pickup D. J. Messenger RNAs of a strongly-expressed late gene of cowpox virus contain 5'-terminal poly(A) sequences. EMBO J. 1987 Dec 1;6(12):3787–3794. doi: 10.1002/j.1460-2075.1987.tb02714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal binding of eucaryotic ribosomes on poliovirus RNA: translation in HeLa cell extracts. J Virol. 1989 Jan;63(1):441–444. doi: 10.1128/jvi.63.1.441-444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Sachs A. B., Davis R. W. The poly(A) binding protein is required for poly(A) shortening and 60S ribosomal subunit-dependent translation initiation. Cell. 1989 Sep 8;58(5):857–867. doi: 10.1016/0092-8674(89)90938-0. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Schrom M., Bablanian R. Inhibition of protein synthesis by vaccinia virus. I. Characterization of an inhibited cell-free protein-synthesizing system from infected cells. Virology. 1979 Dec;99(2):319–328. doi: 10.1016/0042-6822(79)90011-4. [DOI] [PubMed] [Google Scholar]
- Schwer B., Visca P., Vos J. C., Stunnenberg H. G. Discontinuous transcription or RNA processing of vaccinia virus late messengers results in a 5' poly(A) leader. Cell. 1987 Jul 17;50(2):163–169. doi: 10.1016/0092-8674(87)90212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon R., Jurale C., Kates J. Detection of polyadenylic acid sequences in viral and eukaryotic RNA(polu(U)-cellulose columns-poly(U) filters-fiberglass-HeLa cells-bacteriophage T4). Proc Natl Acad Sci U S A. 1972 Feb;69(2):417–421. doi: 10.1073/pnas.69.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon R., Kates J. Mechanism of poly(A) synthesis by vaccinia virus. J Virol. 1974 Aug;14(2):214–224. doi: 10.1128/jvi.14.2.214-224.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Shatkin A. J., Banerjee A. K. Absence of polyadenylic acid from reovirus messenger ribonucleic acid. J Biol Chem. 1973 Dec 10;248(23):7993–7998. [PubMed] [Google Scholar]
- Su M. J., Bablanian R. Polyadenylated RNA sequences from vaccinia virus-infected cells selectively inhibit translation in a cell-free system: structural properties and mechanism of inhibition. Virology. 1990 Dec;179(2):679–693. doi: 10.1016/0042-6822(90)90135-e. [DOI] [PubMed] [Google Scholar]
- Voorma H. O., Goumans H., Amesz H., Benne R. The control of the rate of initiation of eukaryotic protein synthesis. Curr Top Cell Regul. 1983;22:51–70. doi: 10.1016/b978-0-12-152822-5.50006-3. [DOI] [PubMed] [Google Scholar]
- Wright C. F., Moss B. In vitro synthesis of vaccinia virus late mRNA containing a 5' poly(A) leader sequence. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8883–8887. doi: 10.1073/pnas.84.24.8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steeg H., Thomas A., Verbeek S., Kasperaitis M., Voorma H. O., Benne R. Shutoff of neuroblastoma cell protein synthesis by Semliki Forest virus: loss of ability of crude initiation factors to recognize early Semliki Forest virus and host mRNA's. J Virol. 1981 May;38(2):728–736. doi: 10.1128/jvi.38.2.728-736.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]