Abstract
Many “workers” in north temperate colonies of the eusocial paper wasp Polistes fuscatus disappear within a few days of eclosion. We provide evidence that these females are pursuing an alternative reproductive strategy, i.e., dispersing to overwinter and become nest foundresses the following spring, instead of helping to rear brood on their natal nests. A female is most likely to stay and help at the natal nest (i.e., least likely to disperse) when it is among the first workers to emerge and when it emerges on a nest with more pupae (even though worker-brood relatedness tends to be lower in such colonies). The latter cause may result from the fact that pupae-laden nests are especially likely to survive, and thus any direct or indirect reproductive payoffs for staying and working are less likely to be lost. Disappearing females are significantly smaller than predicted if dispersal tendency was independent of body size (emergence order-controlled), suggesting that the females likely to be most effective at challenging for reproductive rights within the natal colony (i.e., the largest females) are also most likely to stay. Thus, early dispersal is conditional on a female’s emergence order, the maturity of its natal nest, and its body size. Finally, we present evidence that foundresses may actively limit the sizes of first-emerging females, perhaps to decrease the probability that the latter can effectively challenge foundresses for reproductive rights. The degree to which foundresses limit the size of first-emerging females accords well with the predictions of the theory of staying incentives.
Recent studies of social insects have revealed surprisingly high levels of plasticity in the expression of altruism, i.e., the amount of reproductive self-sacrifice exhibited by one member of the society on behalf of another. In some termites, for example, workers can facultatively switch from worker morphology and behavior to winged, dispersing reproductive forms (alates) when the resources available to the colony become sufficiently scarce. In addition, even soldiers can become fertile under certain conditions (1). In the eusocial Hymenoptera, membership in either the worker or the reproductive caste is strongly dependent on larval nutrition, which presumably affects the benefits and costs of challenging for reproductive dominance or for breeding solitarily (2). The relative investment by females in worker or queen-associated activities also appears to be affected by their histories of success in competitive interactions with nestmates, with losers increasing their levels of altruistic investment in nestmate relatives (3, 4).
In temperate social wasps of the genus Polistes, females can flexibly assume worker or reproductive roles depending on nutrition, time of year, and social conditions such as the presence of brood (5, 6). In addition, workers produced earlier in the colony cycle sometimes leave their natal colonies and found their own nests solitarily in the same season (7–9), replace the foundress queen (10), or even lay eggs in the presence of the queen (11). In an extreme example of plasticity in altruism, a fraction of first-brood females in the temperate halictid bee, Halictus rubicundus, do not become workers but instead disperse, enter early diapause, and reappear as nest foundresses near their natal sites the following year (12, 13).
The prevalence of facultative, early female dispersal in social insects is unknown, because relatively few field studies couple the systematic marking of potential workers (henceforth described simply as first-brood or “early” adult females) with follow-up censuses of adults founding nests at the females’ natal sites the following year. Moreover, early emerging females that disperse away from their natal sites will be undetected in censuses of the following year’s colonies at these sites. Finally, very little is known about the ecological, social, and genetic factors influencing the decision to become an early disperser, and whether early females respond to these factors in ways that maximize their inclusive fitnesses.
We provide demographic and genetic evidence that a substantial fraction of early females in the temperate, eusocial paper wasp Polistes fuscatus disperse as future nest foundresses instead of staying and helping at their natal nests. Thus, facultative early female dispersal must have evolved independently at least twice in the eusocial Hymenoptera (polistine wasps and halictid bees). In addition, we show how the probability of early dispersal by a female is affected by its emergence order, its body size, the number of foundresses on its natal nest, and its natal nest’s size and maturity. Finally, we provide evidence that foundresses may nutritionally manipulate the adult sizes of the earliest first-emerging females, i.e., females that are likely to stay at the natal nest and become dominant workers.
MATERIALS AND METHODS
Study Populations.
We studied field-nesting Polistes fuscatus colonies at five sites around Ithaca, New York in 1993 and 1994. In our study population, foundresses initiate nests near mid-May (31/107 = 29% of sampled colonies were founded by multiple foundresses in both years), and the first female progeny (potential dispersers or workers) begin to emerge in the first week of July. This worker-production phase proceeds until the first males and gynes (late-season females destined to become foundresses the following spring) begin emerging around 1 August.
In 1993, we studied 15 colonies (9 single-foundress and 6 multiple-foundress colonies) from 23 June to 2 August at two sites separated by approximately 1 km. A total of 21 foundresses and 105 early females (potential workers) were given individually specific paint-marks with Testor’s enamel, and the winglengths of foundresses and their adult offspring were measured (from tegulum to wingtip) as soon as they were first censused. Each colony was censused six times during the study period. On 2 August, the remaining females of surviving colonies (n = 11) were collected and frozen for microsatellite genetic analyses.
On 29 July, 1993, five females were found (accidentally) between the stacked combs of a partly exposed, abandoned Vespula nest. Four wasps had marks of early females that emerged prior to 12 July on colonies within 100 m and were in apparent early diapause, i.e., the wasps were extremely sluggish, as if cooled. These wasps were collected and frozen for genetic analyses.
In 1994, we focused on 47 colonies (31 single-foundress and 16 multiple-foundress colonies) from 2 June to 1 August at four different sites ranging from 1 to 4 km apart. A total of 69 foundresses and 173 early females were paint-marked for individual identification; all foundresses and a subsample of workers from 13 colonies were measured for winglength. The colonies at one site, the Liddell field laboratory (19 colonies), were frequently censused between 2 June and 1 August (36 censuses, with at least 1 day between censuses). Between 6 June and 2 August there were 8 censuses at the other three sites (28 colonies total). On 8 August, the adults present on 12 surviving colonies (4 single-foundress and 8 multiple-foundress) were collected and frozen for microsatellite genetic analyses.
Census Data.
For each colony, we recorded the identifications of all resident adults and marked new adults, noting whether the latter were newly eclosed wasps, as indicated by black eyes. We define a cohort as a group of newly eclosed, early females appearing together for the first time at a census. Cohorts were categorized by their order of emergence, with the first cohort of females being the first censused group of newly eclosed (black-eyed) unmarked females, the second cohort being the next censused group of newly eclosed females, etc. Females of all of the cohorts in our study had been reared by foundresses exclusively (not by workers). Different cohorts emerged within only a few days of each other. For example, second-cohort females emerged on average only 3.4 days later than first-cohort females. Thus, first-cohort workers played no role in the second cohort’s rearing because the latter were already at the pupal stage (when no feeding occurs).
The nest tenure for each early female, i.e., the number of days each female spent on nest, was estimated for the intensively censused 1994 Liddell colonies as follows. The female’s first day was calculated as the midpoint between the census day before the female was first seen and the census day that the female was first seen and marked; the female’s last day was calculated as the midpoint between the census day it was last seen and the first census day after its (permanent) disappearance. Nest tenure was then estimated as the difference between the female’s last and first days. Nest size was measured as the total number of brood cells on 30 June, which is just prior to initial worker emergence. Overall nest maturity was measured as the number of pupae divided by the total number of brood cells on the same date.
As a measure of the propensity of early females to disappear from a colony, we calculated the proportion of females eclosing on or prior to 12 July (for 1993 colonies) or 8 July (for 1994 colonies) that remained with the colony until the end of the worker phase on 1 August.
Genetic Analyses.
After screening microsatellite primers derived from Polistes and Parachartergus wasps sent to us by Joan Strassmann and David Queller (Rice University), we found eight primers detecting polymorphic loci having from 4- to 21-length alleles and with heterozygosities ranging from 0.60 to 0.91. The primer loci with corresponding mean heterozygosity and number of alleles are, respectively: Pbe128TAG, 0.61, 6; Pbe269bAAG, 0.82, 12; Pbe411AAT, 0.84, 9; Pbe424AAT, 0.86, 12; Pbe440AAT, 0.91, 21; Pbe442AAT, 0.70, 8; Paco3155TAG, 0.65, 6; Paco3219AAG, 0.60, 4.
We used the methods of Choudhary et al. (14) to obtain microsatellite genotypes at all eight loci for a total of 98 early females from 11 1993 colonies (7 single-foundress and 4 multiple-foundress) and 12 1994 colonies (4 single-foundress and 8 multiple-foundress). We used the relatedness program (version 4.2) of Goodknight and Queller (15), based on the logic of Queller and Goodnight (16), to estimate the mean relatedness among wasps.
Statistical Methods.
Parametric tests were used only when assumptions of normality appeared satisfied. Proportions of workers staying on the nest were arcsine square root-transformed for all statistical tests. A three-way analysis of variance of the effect of site, foundress number, and foundress presence (i.e., whether at least one foundress remained on the nest until 1 August) was performed on the transformed proportion of staying workers. For this analysis, the five sites were combined into two sites (the five sites fell into two spatial clusters >1 km apart) to obtain a more balanced design. Early female tenures measured in the 1994 Liddell colonies were square root-transformed to yield approximate normality for parametric tests. All tests are two-tailed.
RESULTS
Genetic Data.
The mean relatedness among single-foundress early females (sampled 2–8 August) was 0.74 ± SE 0.04, which is close to the theoretical value of 0.75 for singly mated, outbreeding queens (n = 11 colonies). In multiple-foundress colonies, the mean relatedness among early females (sampled 2–8 August) was only 0.38 ± 0.04 (n = 11 colonies with multiple early females), which is significantly less than that for single-foundress colonies (P = 0.0002; two-tailed Mann–Whitney U test). The latter result suggests that cofoundresses share in reproduction.
Evidence of Early Female Dispersal.
In 1993 and 1994, four unambiguously identifiable females that emerged between 3 and 12 July permanently disappeared from their natal nests within 3 days of eclosion. Three of the females were later found sitting on inactive combs (two on 22 July, 1993, one on 7 September, 1994, on nests >10 m from their natal nests), and one female was on a distant active nest late in the season (7 September 1994 on a nest >100 m from the natal colony). Late in the season, the mixing of gynes (future foundresses) from different natal colonies on the few remaining active large colonies is common, as these females attempt to parasitize dwindling resources from the few colonies still possessing some foraging workers (Reeve, unpublished data).
In 1994, a female that emerged on 8 July disappeared by 13 July and returned on 16 May, 1995 to singly found a new nest near its natal nest. [A second probable case of spring nest-founding by an early female marked the previous summer was documented in 1997, and a third case has been observed in a Michigan population of P. fuscatus (G. Gamboa, personal communication)].
On 29 July, 1993, five females were found between the stacked combs of a partly exposed, abandoned Vespula nest. The wasps, four of which had the same marks as females disappearing from nests within 100 m prior to 12 July 1993, were lethargic and apparently in diapause. The females had a low mean relatedness to each other (r = 0.138, SE = 0.13), but two of them (likely full sisters) had identical genotypes (r = 1.00) that closely matched the genotype of a foundress in a multiple-foundress colony about 30 m away (mean relatedness to foundresses = 0.54). Indeed, females with marks exactly matching those of the diapausing wasps had disappeared from the latter colony by 12 July. The other two marked females could not be matched genetically to their natal colonies because there were no adults in several (vacated) colonies at the time of collection, but their marks corresponded to the marks of missing early females (and not foundresses or gynes) from colonies in the area. (In July, 1998, two more disappearing, first-cohort, marked females were recovered in a diapause aggregation with unmarked females approximately 2 m from the natal colony.)
Overall, 10 of 278 marked early females in 1993–1994 (3.6%) disappeared from their natal nests shortly after eclosion and were later recovered in contexts associated with overwintering and nest founding in the following season. Nearly 75% of early females disappeared during the worker phase (Fig. 1). The low percentage of disappearing early females that were later recovered (5%) may greatly underestimate the actual percentage of early dispersers (as opposed to workers dying while working for their colonies) if such females typically disperse out of their natal sites (before or after overwintering) or have high mortality rates. Both population-genetic and mark-and-recapture evidence for our population indicate that dispersal out of the immediate vicinity of the natal nest before nest founding and/or a high mortality rate also is typical of late-season females (gynes) known to become foundresses the following spring. For example, only 11 of 130 or 9% of fall gynes (females emerging after 1 August) marked in 1994 at Liddell colonies were recovered as foundresses at the same site the following spring, in contrast to many Polistes populations in which marked foundresses typically return with high probability to found nests near their natal nests (17).
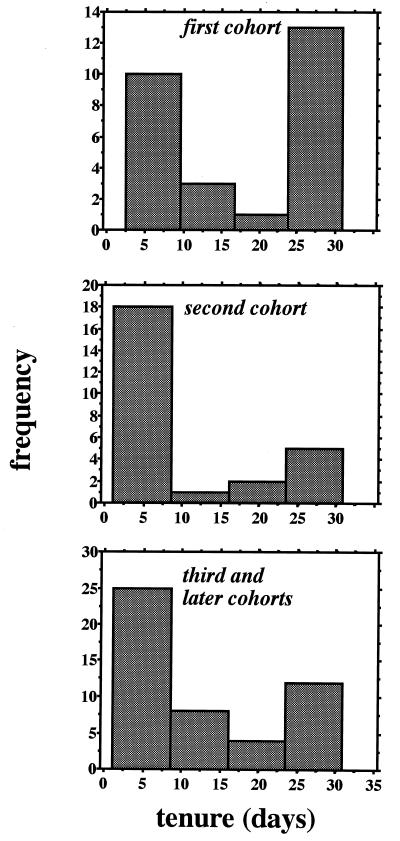
Figure 1.
Frequency distribution of tenures for first-, second-, and later-cohort early females (data from pooled 1994 Liddell colonies).
Determinants of Early Female Nest Tenure.
Early females frequently disappeared from nests shortly after eclosion: 47% of first- and second-cohort wasps and 31.5% of last-cohort wasps had mean nest tenures of less than 10 days post-eclosion (mean tenures calculated by cohort across 1994 Liddell colonies). Nearly one-third (29%) of all early females had tenures of less than 5 days post-eclosion (1994 Liddell colonies). The overall frequency distribution of early female tenures (1994 Liddell colonies pooled) is strongly bimodal, with most females either disappearing within 5 days of emergence or staying at their natal colonies for the entire worker phase and beyond (≥30 days; Fig. 1). These frequency distributions are markedly different from the monotonically decreasing, negative exponential distributions expected if there was a constant probability of disappearance per unit time.
A three-way ANOVA controlling for site effects shows that early females were (i) more likely to disappear from single-foundress colonies than from multiple-foundress colonies and (ii) more likely to disappear from colonies without foundresses than from colonies with at least one foundress present (Table 1; 1993 and 1994 data on proportion of staying workers combined).
Table 1.
Proportions of early females staying on the nest throughout the worker phase as a function of foundress presence and initial foundress number (n = 45; 1993 and 1994 colonies combined)
| Foundress status | Proportion of early females staying on nest
|
|
|---|---|---|
| Single-foundress | Multiple-foundress | |
| Absent | 0.098 | 0.109 |
| Present | 0.112 | 0.259* |
Three-factor ANOVA (site, foundress presence, foundress number) revealed a significant positive effect of foundress presence (F test = 7.70; P = 0.0086) and a significant positive effect of foundress number (F test = 7.66; P = 0.0088).
For the 1994 Liddell data only, for which we had detailed data on tenures of marked early females (see Materials and Methods), we performed a two-factor ANOVA on early female tenure versus foundress number (multiple or single) and presence or absence of the foundress(es). For the first female cohort, the results were similar to the above: mean tenures were significantly longer in multiple-foundress than in single-foundress colonies (P = 0.02) and also marginally significantly longer in colonies with foundresses present versus those without foundresses present throughout the entire worker phase (P = 0.05; mean-square-root tenures of first cohort = 2.96 for single-foundress colonies without a foundress, 4.07 for single-foundress colonies with a foundress, 4.41 for multiple-foundress colonies with no foundresses, and 6.40 for multiple-foundress colonies when at least one foundress remained). However, there were no such relationships for the second-female cohort (P = 0.70 for queen-number effect and P = 0.33 for foundress-presence effect; mean-square-root tenures of first cohort = 3.79 for single-foundress colonies without a foundress, 3.90 for single-foundress colonies with a foundress, 2.41 for multiple-foundress colonies with no foundresses, and 4.45 for multiple-foundress colonies when at least one foundress remained).
The overall mean early female tenure in single-foundress colonies was 13.41 days (SD = 9.33) for the first cohort and 17.27 (SD = 12.87) days for the second cohort. The overall mean early female tenure in multiple-foundress colonies was 30.18 days (SD = 17.09) for the first cohort and 15.75 (SD = 20.36) days for the second cohort. The difference in square root-transformed tenures between first and second cohorts in single-foundress colonies was significantly less than that in multiple-foundress colonies (P = 0.02, Student’s t test). Thus, the first emerging workers in multiple-foundress colonies were especially likely to stay; all others tended to disappear at similarly high rates.
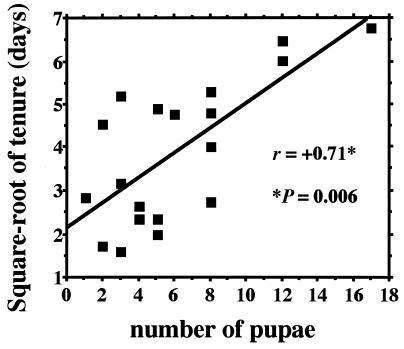
To determine the most important proximate factors in a worker’s decision to stay rather than to disperse, we performed a multiple regression of the square root of the tenure of the first cohort (1994 Liddell colonies) as a function of four (intercorrelated) variables that are likely assessable by workers: queen number, foundress presence (1 = present; 0 =absent), comb size on 30 June (just prior to emergence of first females), and nest maturity on 30 June. Only the nest maturity was a significant predictor of worker staying (partial regression coefficient = 6.07; P = 0.01); queen number, foundress presence, and comb size (P = 0.45, 0.13, and 0.64, respectively) exerted effects only indirectly through their correlations with mean brood maturity. Although mean brood maturity was the single most important correlate of worker tenure, more mature combs did tend be larger. Indeed, the number of pupae (i.e., the product of mean brood maturity and cell number) was strongly positively correlated with the mean tenure of the first worker cohort (Fig. 2). Thus, it appears that first-emerging workers stay only if the proportion or number of pupae in their colonies is sufficiently large. Because there are more pupae in multiple than in single-foundress colonies, and because foundresses are more likely to disappear from nests with few pupae, such a staying rule explains why workers are most likely to stay and help in multiple-foundress colonies with at least one foundress present.
Figure 2.
Correlation between early-female tenure in days (square root-transformed) and number of pupae at initial worker eclosion (1994 Liddell colonies).
Worker Size and Dispersal.
The mean sizes (winglengths) of staying and leaving early females in single-foundress colonies (1.31 cm ± 0.02 SE and 1.25 cm ± 0.02 SE, respectively) were significantly less than the corresponding mean sizes of early females in multiple-foundress colonies (1.39 cm ± 0.020 SE; P = 0.01 and 1.34 cm ± 0.02 SE; P = 0.028, respectively; two-tailed t tests; 1993 and 1994 colonies).
First-cohort females had significantly smaller winglengths (mean = 1.29 cm ± 0.02 SE) than second- or later-cohort females (mean = 1.34 cm ± 0.02 and 1.36 cm ± 0.02, respectively; P < 0.0001, repeated-measures ANOVA, n = 18 1993 and 1994 colonies with measured workers from at least three cohorts). The latter two cohorts did not differ significantly in mean size.
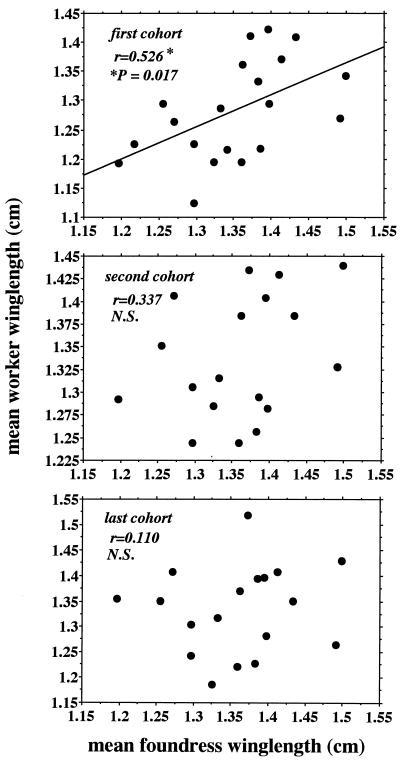
First-cohort mean female size was significantly positively correlated with the mean size of their foundress(es) in all 1993 and 1994 colonies for which both sizes were available (Fig. 3). However, despite the fact that later-female cohorts also had been exclusively reared by foundresses, there was no significant correlation between the mean size of second- or later-cohort females and the mean size of their foundress(es) (Fig. 3). In single-foundress colonies, the association between first-cohort female mean size and foundress size was positive but not significant (n = 10 colonies; regression slope = +0.37; P = 0.28); however, this association was both positive and significant in multiple-foundress colonies (n = 10 colonies; regression slope = +0.73; P = 0.04).
Figure 3.
(Top) Correlation between mean size of first cohort of early females and mean size of foundresses. (Middle) Correlation between mean size of second cohort of early females and mean size of foundresses. (Bottom) Correlation between mean size of last cohort of early females and mean size of foundresses. n = 18–20 colonies (1993 and 1994 Liddell colonies for which both foundress and first- and later-cohort winglength data were available).
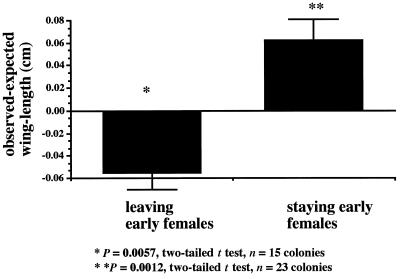
Early females that permanently left the nest were, on average, significantly smaller than the size expected if leaving was independent of size (Fig. 4; expected size = mean size of all second-and later-cohort females, because second- and later-cohort females were most likely to leave). Conversely, staying females were significantly larger than expected if leaving was independent of size (Fig. 4; expected size = mean size of all first-cohort females, because first-cohort females were most likely to stay). In addition, the correlation between the square root-transformed mean tenure for first-cohort females and mean foundress size was significantly negative (r = −0.52; P = 0.026; n = 19 1994 Liddell colonies).
Figure 4.
Mean observed minus expected winglengths for early females that disappeared from or stayed at the colony.
DISCUSSION
Our evidence indicates that many early disappearing “workers” are pursuing selfish reproductive options, e.g., overwintering to become foundresses the following year, instead of helping to rear brood on their natal nests. The probability of early female dispersal is affected by the state of its colony. A first-eclosing female is most likely to stay and help at the natal nest when the female (i) is large and (ii) is among the first to emerge on a colony founded by multiple foundresses (despite the lower mean relatedness to brood in single- versus multiple-foundress colonies). A partial regression analysis indicates that the proportion or total number of pupae on the natal nest may be the single most important proximate determinant of the decision to stay and help.
Why are early females more likely to help on nests with more pupae despite their lower relatedness to nestmates on such nests? We propose three hypotheses. (i) Workers are more likely to escape queen reproductive policing on larger nests. However, this hypothesis by itself does not explain why females work, and, moreover, existing data suggest that worker production of reproductives in P. fuscatus colonies with foundresses present is rare (18). (ii) Early females prefer to become workers on larger, more mature nests because more resources (per adult) that are critical for overwintering can be obtained on such nests. However, the latter benefit is devalued to the extent that risky or energetically costly helping itself reduces the probability of successful overwintering (5, 6, 8). (iii) More mature nests will produce more nest-tending adults sooner and thus are less likely to fail from chance loss of all nest-tending adults. Indeed, this mortality factor generates the principal advantage for nest cofounding by multiple foundresses in our study population (19). Thus, in accordance with Queller’s (20, 21) “head-start” or survival-insurance hypothesis, workers prefer to stay and help on larger, more mature natal nests (which will soon have additional helping adults) because the worker’s direct or indirect reproductive payoffs are less likely to be lost through colony failure. This hypothesis also explains the otherwise puzzling observation that the proportion or number of pupae may be the most potent single proximate determinant of worker staying.
Why are earlier emerging females more likely to become workers? Because older workers generally are dominant to younger workers in Polistes (reviewed in ref. 22), it follows that first-emerging workers would have a relatively high direct reproductive stake in the natal colony [either via reproduction in the presence of the queen or through queen supersedure, the latter being well documented (reviewed in ref. 22)]. This greater opportunity for personal reproduction in the natal colony may make staying favorable for earlier emerging but not later emerging females. Alternatively, slightly later emerging females may be more likely to disperse because the prior presence of workers diminishes the degree to which these females could further improve colony success—as when colony success is a concave function of the number of helping workers (23).
Departing early females are significantly smaller than expected if dispersal tendency was independent of body size. Conversely, staying workers are larger than expected (emergence-order controlled; Fig. 4). These results make sense if a larger worker is more effective at challenging foundresses for reproductive rights within its natal nest and thus benefits more from staying. In support of this hypothesis, first-cohort early females had shorter mean tenures when their foundresses were larger (see Results).
Intriguingly, our evidence suggests that foundresses nutritionally manipulate (reduce) the sizes of early females that are most likely to become reproductive threats. First-emerging early females, which are likely to be dominant over younger workers if they remain at the natal nest (reviewed in ref. 22), are significantly smaller than later-emerging early females. The latter result may reflect foundress regulation of brood nutrition to ensure that first-emerging workers are not too large and thus not too effective at challenging for reproductive rights.
It may be argued that the smaller size of earlier emerging workers is simply a reflection of a passive tendency for smaller workers to develop faster or a lower food availability for the first brood. However, the involvement of the foundress in precisely regulating the sizes of the early females is suggested by the significant, positive correlation between the mean size of the foundress(es) and their first-emerging workers, with mean foundress size consistently exceeding worker size (Fig. 4). Importantly, there is no such significant correlation between sizes of foundress(es) and sizes of slightly later emerging females, the latter often being larger than the foundress(es) (Fig. 4); these later emerging females, like wasps of all cohorts in our study, had been reared exclusively by foundresses. Together, these data suggest that foundresses nutritionally ensure that first-emerging females are no larger than some threshold size that is positively related to the sizes of the foundresses themselves. By limiting a first-emerging female’s size, the foundress can lower the relative fighting ability of a female likely to become a dominant worker, thereby reducing the effectiveness of reproductive challenges from that female or the size of any peace incentives conceded to the female. Because first-emerging females are most likely to become highly ranked workers (reviewed in ref. 22), foundresses may be favored to manipulate only the earliest-developing brood.
The degree to which foundresses reduce the size of first-emerging females should be constrained by the greater propensity of smaller females to leave the colony altogether (see above). In particular, foundresses should reduce the first-emerging female’s size just to the point at which leaving the colony would become favored for the latter. This theoretical minimal worker size can be derived from the theory of staying incentives (25, 26), if we assume that the probability of direct reproduction by a worker is an increasing function p(sw/sf) of the ratio of the worker’s size sw to the controlling foundress’s size sf. Let r be the potential worker’s mean relatedness to the foundress’s offspring, divided by its relatedness to its own offspring (27). Let x be the expected success of a dispersing early female and k be the colony success if the potential worker stays, both standardized relative to a success of 1.0 for the foundress if the early female disperses. The foundress should then reduce the early female’s size just to the point at which
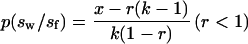 |
1 |
(see refs. 26 and 27). (Note: The foundress benefits from retaining an early female as a worker if x < k − 1.) If the foundress’s optimal ratio sw/sf equals a*, then it follows that the size of the first-cohort female should be linearly related to the size of the foundress, i.e., sw = a*sf. Moreover, according to Eq. 1, the slope a* should be higher in multiple-foundress colonies (lower r) than in single-foundress colonies (higher r), exactly as observed (see Results). The model further predicts that early females in multiple-foundress colonies should be larger than those in single-foundress colonies, which also was observed (see Results). Thus, our data are consistent with the staying-incentive model of worker-size manipulation by foundresses.
The remarkable behavioral plasticity exhibited by early P. fuscatus females reinforces growing evidence that social behavior is strongly context-dependent, even in insects (24). In addition, the ability of early females to disperse as future foundresses has crucial implications for tests of sex ratio and “optimal skew” theory. For tests of sex ratio theory, early female dispersal means that previous empirical studies have probably underestimated the degree to which social wasp sex-investment ratios are female-biased and thus are an expression of worker versus queen genetic interests (28). For tests of optimal skew theory (25, 26), it now becomes necessary to consider the possibility that dominant foundresses allow subordinates to produce reproductively valuable “workers” as a direct incentive to cooperate in a multiple-foundress association.
Acknowledgments
We thank Lee Dugatkin and George Gamboa for valuable comments. The 1998 early-female diapause aggregation was discovered by Sandra Nasrallah. H.K.R. and P.N. were supported by a National Science Foundation grant; H.K.R. was also partly supported by a New York State Hatch grant.
References
- 1.Shellman-Reeve J. In: Social Behavior in Insects and Arachnids. Choe J, Crespi B, editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 52–93. [Google Scholar]
- 2.Wheeler D E. Am Nat. 1986;128:13–34. [Google Scholar]
- 3.West-Eberhard M J. Science. 1967;157:1584–1585. doi: 10.1126/science.157.3796.1584. [DOI] [PubMed] [Google Scholar]
- 4.West Eberhard M J. In: Natural Selection and Social Behavior. Alexander R D, Tinkle D W, editors. New York: Chiron; 1981. pp. 3–17. [Google Scholar]
- 5.Solis C R, Strassmann J E. Funct Ecol. 1990;4:531–542. [Google Scholar]
- 6.Mead F, Pratte M, Gabouriaut D. Insectes Soc. 1993;37:236–250. [Google Scholar]
- 7.Strassmann J E. Behav Ecol Sociobiol. 1981;8:55–64. [Google Scholar]
- 8.Mead F, Gabouriaut D. Insectes Soc. 1995;42:385–396. [Google Scholar]
- 9.Page R E, Post D C, Metcalf R A. Am Nat. 1989;134:731–748. [Google Scholar]
- 10.Queller D C, Peters J M, Solis C R, Strassmann J E. Behav Ecol Sociobiol. 1997;40:3–16. [Google Scholar]
- 11.Miyano S. Res Pop Ecol. 1986;28:347–361. [Google Scholar]
- 12.Yanega D. Proc Natl Acad Sci USA. 1988;85:4374–4377. doi: 10.1073/pnas.85.12.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanega D. Behav Ecol Sociobiol. 1989;24:97–107. [Google Scholar]
- 14.Choudhary M, Strassmann J E, Solis C R, Queller D C. Biochem Genet. 1993;31:87–96. doi: 10.1007/BF02399822. [DOI] [PubMed] [Google Scholar]
- 15.Goodknight, K. F. & Queller, D. C. (1994) Goodknight Software.
- 16.Queller D C, Goodknight K F. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. [DOI] [PubMed] [Google Scholar]
- 17.Klahn J E. Behav Ecol Sociobiol. 1979;5:417–424. [Google Scholar]
- 18.Metcalf R A. Am Nat. 1980;116:642–654. [Google Scholar]
- 19.Reeve H K, Nonacs P. Behav Ecol. 1997;8:75–82. [Google Scholar]
- 20.Queller D C. Proc Natl Acad Sci USA. 1989;86:3224–3226. doi: 10.1073/pnas.86.9.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Queller D C. Proc R Soc London Ser B. 1994;256:105–111. [Google Scholar]
- 22.Reeve H K. In: The Social Biology of Wasps. Ross K, Matthews R, editors. Ithaca, NY: Cornell Univ. Press; 1991. pp. 99–148. [Google Scholar]
- 23.Nonacs P. Oikos. 1991;61:122–125. [Google Scholar]
- 24.Nonacs P, Reeve H K. Ecology. 1995;76:953–967. [Google Scholar]
- 25.Vehrencamp S L. Anim Behav. 1983;31:667–682. [Google Scholar]
- 26.Reeve H K, Ratnieks F A. In: Queen Number and Sociality in Insects. Keller L, editor. Oxford, U.K.: Oxford Univ. Press; 1993. pp. 45–85. [Google Scholar]
- 27.Reeve H K, Keller L. Am Nat. 1995;145:119–132. [Google Scholar]
- 28.Noonan K M. Science. 1978;199:1354–1356. doi: 10.1126/science.199.4335.1354. [DOI] [PubMed] [Google Scholar]