Abstract
Two potential outcomes of a coevolutionary interaction are an escalating arms race and stable cycling. The general expectation has been that arms races predominate in cases of polygenic inheritance of resistance traits and permanent cycling predominates in cases in which resistance is controlled by major genes. In the interaction between Depressaria pastinacella, the parsnip webworm, and Pastinaca sativa, the wild parsnip, traits for plant resistance to insect herbivory (production of defensive furanocoumarins) as well as traits for herbivore “virulence” (ability to metabolize furanocoumarins) are characterized by continuous heritable variation. Furanocoumarin production in plants and rates of metabolism in insects were compared among four midwestern populations; these traits then were classified into four clusters describing multitrait phenotypes occurring in all or most of the populations. When the frequency of plant phenotypes belonging to each of the clusters is compared with the frequency of the insect phenotypes in each of the clusters across populations, a remarkable degree of frequency matching is revealed in three of the populations. That frequencies of phenotypes vary among populations is consistent with the fact that spatial variation occurs in the temporal cycling of phenotypes; such processes contribute in generating a geographic mosaic in this coevolutionary interaction on the landscape scale. Comparisons of contemporary plant phenotype distributions with phenotypes of herbarium specimens collected 9–125 years ago from across a similar latitudinal gradient, however, suggest that for at least one resistance trait—sphondin concentration—interactions with webworms have led to escalatory change.
Coevolution was usefully defined by Janzen (1) as “an evolutionary change in a trait of the individuals in one population in response to a trait of the individuals of a second population, followed by an evolutionary response by the second population to the change in the first.” Documenting such reciprocity of responses, particularly between insect herbivores and their host plants, has proved difficult; Thompson (2) identifies one major obstacle to such demonstration as the dearth of testable hypotheses associated with coevolutionary theory. The two most widely predicted outcomes of coevolutionary interactions are escalating arms races, resulting from directional selection, and stable or fluctuating polymorphisms, maintained by frequency-dependent or density-dependent selection. Arms races are the expected outcome of interactions governed by polygenic traits, whereas cycles are expected in cases in which resistance is caused by the action of major genes (2). Seger (3), in fact, argues that stable cycling is extremely unlikely for species interacting via changes in polygenic traits, at least in part because under these conditions rare phenotypes cannot increase in frequency unless the mean of the population is under selection in the direction of that phenotype. The most compelling examples of reciprocal evolutionary change between interacting insect herbivores and plants to date involve single-locus traits; these systems parallel the gene-for-gene interactions that occur between pathogenic microbes and their host plants. Reciprocal responses in multilocus traits have proved more difficult to document. In the interaction between Depressaria pastinacella, the parsnip webworm, and its principal host plant, Pastinaca sativa, plant-resistance traits as well as insect “virulence” traits display continuous variation, and we demonstrate that within populations a remarkable degree of phenotype frequency-matching, suggestive of stable cycling, occurs between herbivore and host plant.
Wild parsnip, P. sativa, is an introduced European weed naturalized throughout much of eastern North America and found in disturbed habitats. Like many apioid umbellifers, P. sativa produces furanocoumarins, broadly biocidal compounds that owe their toxicity to their ability to crosslink DNA and interfere with transcription (4). Furanocoumarin production is under genetic control, with heritabilities for individual compounds ranging from 0.54 to 0.62, and is responsive to selection by herbivores (5). The principal (and, for much of its range in North America, the only) herbivore associated with P. sativa is the parsnip webworm, D. pastinacella, like its host introduced from Europe and established widely across eastern North America. Throughout its range in the U.S., the insect feeds exclusively on the reproductive structures of P. sativa and, where they are available, species in the genus Heracleum. The ability of parsnip webworms to exist on wild parsnip is caused in part by cytochrome P450-mediated metabolism of furanocoumarins; in this insect, heritabilities for P450 activity levels range from 0.33 to 0.45 (6) and also are subject to selection (7). Thus, the conditions for reciprocal selection exist: genetic variability in the plant’s ability to reduce the impact of the interaction (“resistance”) and in the herbivore’s ability to increase the impact of the interaction (“virulence”). We set out to evaluate the degree of correspondence of plant and herbivore phenotypes by comparing resistance (the capacity to produce furanocoumarins) in four populations of wild parsnip with virulence (the capacity to detoxify furanocoumarins) in four corresponding populations of parsnip webworms. To examine the stability of this correspondence over time, we compared contemporary plant phenotype distributions with those of herbarium specimens collected 9–125 years ago from the same geographic range.
MATERIALS AND METHODS
Plant Chemistry.
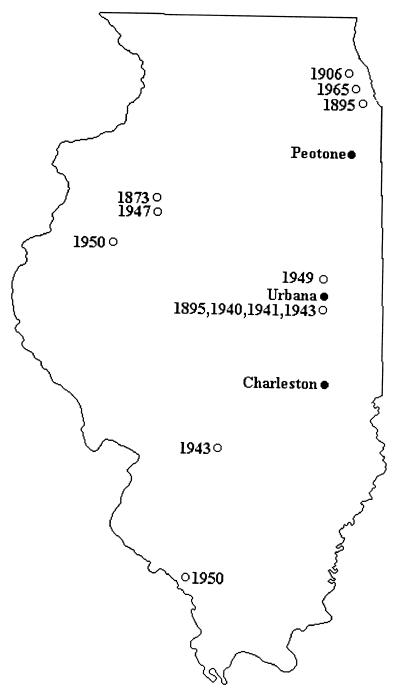
We examined four populations of webworms and wild parsnips along a latitudinal gradient extending from 39.5° to 44° N. For each population, we determined the furanocoumarin profile of plants and the cytochrome P450 capabilities of the insects. Webworm pupae and ripe parsnip seeds were collected near four midwestern towns: Winona (Winona County), MN; Peotone (Will County), IL; Urbana (Champaign County), IL; and Charleston (Coles County), IL. The seeds were weighed and analyzed for content of four furanocoumarins—bergapten, xanthotoxin, isopimpinellin, and sphondin. These furanocoumarins are the most abundant and predictably encountered compounds in the plant. Seeds of 26 plants from each population were analyzed for furanocoumarin content by cutting across the furanocoumarin-containing vittae of a preweighed sample of eight seeds from each plant. The seed halves then were extracted with 2 ml of ethyl acetate, and the extract was analyzed by HPLC as described (8) with the exception that the solvent consisted of 55% cyclohexane, 42% isopropyl ether, and 3% butanol, and an Alltech Associates Adsorbosphere silica 5 μm × 150 mm column was used. For the purpose of comparing present-day chemical profiles with historical chemical profiles, we obtained and analyzed two seeds from each of 19 plant specimens contained in the collection of the Herbarium of the University of Illinois. The specimens all were collected from Illinois (Fig. 1), and dates of collection spanned the years 1873 to 1989.
Figure 1.
Collection year and location of herbarium specimens of wild parsnip from which seed furanocoumarins (isopimpinellin, xanthotoxin, and sphondin) were quantified, as well as locations of Illinois populations included in the study. Not shown are three specimens collected in 1889, 1916, and 1989, respectively, with origin labeled simply as “Illinois” and one specimen (1941) with origin labeled simply as “Michigan”.
Webworm Metabolism.
Because only the immature stages of D. pastinacella feed on parsnip reproductive tissues, the adults that emerged from the pupae collected had to be mated to produce larvae that could be assayed for detoxification capacity. Adults were exposed to an artificial winter to induce mating (9) by maintaining them in a cold room at 10°C and a short daylength (12 h) for approximately 14 wk. For each population, “overwintered” adults were placed together with a parsnip plant inside a screened cage located in a greenhouse in which temperature was set at 30°C and daylength at 16 h. Leaves containing eggs were removed periodically, and neonate larvae were transferred to artificial diet and reared in an insectary maintained at the same temperature and daylength as the greenhouse. Matings within a population were allowed to proceed inside a cage containing no fewer than 50 adults. Larvae that hatched from the eggs resulting from these matings were reared to ultimate (sixth) instar on an artificial diet (9).
To ensure that we would measure the maximum capacity of the webworms to detoxify parsnip furanocoumarins, we transferred the freshly molted sixth-instar larvae to a diet containing 0.1% (fresh weight) xanthotoxin, an amount sufficient to induce furanocoumarin detoxification to high levels (6). After 2 days of feeding on this diet, 25 larvae from each population were sacrificed and their guts removed and prepared for in vitro assay of metabolism of the same four furanocoumarins quantified in the host plants. Guts were homogenized in buffer with a Tissue Tearor (Biospec Products, Bartlesville, OK) according to Berenbaum and Zangerl (6), except that a mixture of the four furanocoumarins in proportions equivalent to the average proportions measured across the four plant populations was used as the substrate.
Statistical Methods.
Mean levels of furanocoumarin metabolism by insects and production by plants were compared among populations by one-way ANOVA. A cluster analysis program (Proc fasclus Version 6.12, SAS Institutes, Cary, NC) was used to identify multivariate phenotypes in both plants and insects. Before cluster analysis, insect metabolism rates were standardized to a mean of one by dividing each individual value by the raw mean value across all populations. The same type of standardization was performed separately for the furanocoumarin content of plants. To assess the level of phenotype matching between insects and plants where phenotypes were defined by cluster analysis, we compared frequencies of insect metabolism phenotypes to frequencies of plant production phenotypes in each population by likelihood-ratio χ2 analysis [SPSS (Chicago) crosstabs, version 7.5.1].
RESULTS AND DISCUSSION
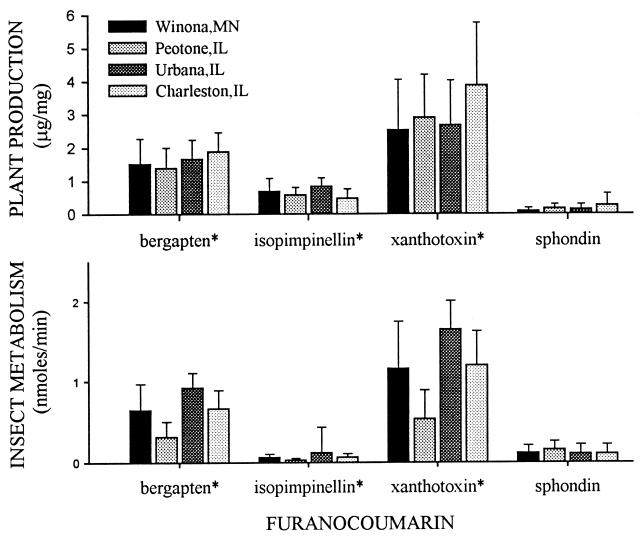
Significant differences were found among plant populations (P < 0.05) in furanocoumarin content and among insect populations (P < 0.05) in metabolic capabilities (Fig. 2). Overall, patterns of furanocoumarin metabolism by webworms matched patterns of furanocoumarin production in the plants; plant production and insect metabolism of bergapten and xanthotoxin were high, whereas production and metabolism of isopimpinellin and sphondin were low (Fig. 2). Similarities in average patterns of furanocoumarin metabolism by insects and production by plants among populations, however, are difficult to interpret. Such patterns provide no information on the degree to which individual insect populations are adapted to the individual plant populations on which they feed. Averages are derived from a collection of phenotypes, and the average insect phenotype is unlikely to be successful on all plant phenotypes, just as the average plant phenotype is unlikely to be equally resistant to all insect phenotypes.
Figure 2.
Mean (±SD) furanocoumarin content (μg/mg) of wild parsnip seeds and furanocoumarin metabolism (nmol/min) by parsnip webworm larvae from four midwestern U.S. populations. Furanocoumarins for which significant differences were found between populations by one-way ANOVA (P < 0.05) are indicated by ∗.
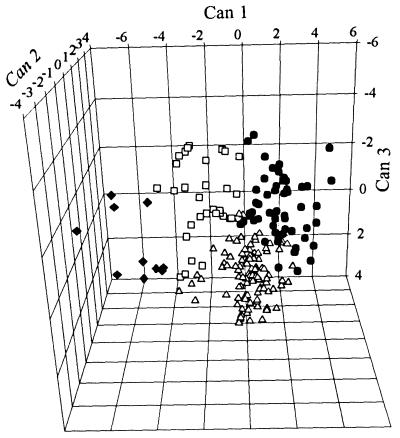
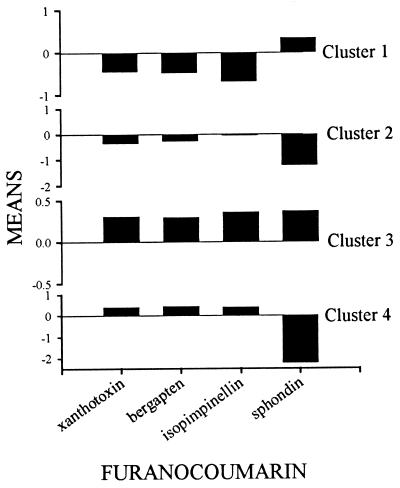
Previous studies of a population in Champaign County (Urbana, IL) revealed the existence of substantial genetic variation in both the rate of detoxification of furanocoumarins in webworms (6) and the amount of production of furanocoumarins by parsnips (5). Because this variation is quantitative in nature, individuals cannot be assigned to discrete phenotypes. Rather, we performed cluster analysis to identify groups of chemically similar phenotypes. The program was instructed to construct four clusters based on the standardized data. Contrary to expectation, population origin did not delimit the four clusters (Fig. 3); that is to say, each of the four clusters did not contain insects and plants from a single population. Instead, each cluster contained individual plants and insects from all four populations. Each cluster thus describes a multitrait phenotype that occurs at some frequency in all or most of the populations. Both plant and insect phenotypes reflect relative variation in production (in the case of the plants) and metabolism (in the case of the insects) of angular (sphondin) and linear (xanthotoxin, bergapten, and isopimpinellin) furanocoumarins (Fig. 4). In the plant, the basis for this variation likely is caused by a biosynthetic dichotomy in production; the two classes of furanocoumarins result from an early branch in the furanocoumarin pathway (4). The basis for the variation in the insects is not clear but may reflect differential substrate specificity of multiple cytochrome P450s (6).
Figure 3.
Three-dimensional representation of the four clusters including both plant and insect phenotypes (each point represents a single plant or insect). Cluster analysis was performed to identify groups of chemically similar phenotypes in webworm detoxification and plant furanocoumarin production. Before cluster analysis, insect metabolism rates and plant furanocoumarin contents were standardized to a mean of one by dividing each individual value by the raw mean value across all populations. Clusters were produced by Proc fasclus Version 6.12 (SAS Institutes, Cary, NC). Proc candisc was used to generate three canonical variables (CAN1, CAN2, and CAN3) from the cluster analysis output for use in plotting the four clusters in three-dimensional space.
Figure 4.
Characterization of the four clusters shown in Fig. 2 in terms of average furanocoumarin content (plants) and average furanocoumarin metabolism (insects).
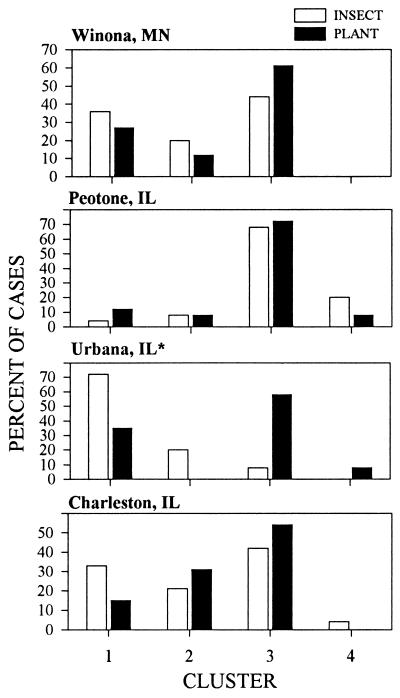
When the frequency of plant phenotypes belonging to each of the clusters is compared with the frequency of the insect phenotypes belonging to each of the clusters in the different populations, an extraordinary degree of frequency matching is revealed in three of the populations (Fig. 5). Not only is the probability of matching chemical phenotypes high within populations, it is low between populations (Table 1), and thus is not attributable to the overall similar patterns of production and metabolism evident in Fig. 2. Phenotype matching by the insect to the plant hosts was not diminished by recombination resulting from sexual reproduction; webworm offspring phenotypes matched parental host plant phenotypes, despite the fact that sexual reproduction by the webworms might have been expected to diminish or even eliminate matching. The high level of matching between offspring phenotypes and those of parental hosts suggests that the genes conferring ability to exploit hosts, i.e., the ones that code for cytochrome P450s, are tightly linked, perhaps as a result of gene duplication and divergence.
Figure 5.
Phenotype frequency distributions of insects and plants for each of the four populations. The χ2 values are from likelihood-ratio estimates testing the hypothesis that the two distributions are the same (See Table 1); ∗ denotes a significant mismatch between plant and insect patterns.
Table 1.
Comparisons between insect and plant populations for goodness of fit
| Plant origin | Insect origin
|
|||
|---|---|---|---|---|
| Winona | Peotone | Urbana | Charleston | |
| Winona | 0.434 | 0.030 | 0.016 | 0.222 |
| Peotone | 0.493 | 0.009 | 0.005 | |
| Urbana | <0.000 | <0.000 | ||
| Charleston | 0.258 | |||
Values are probabilities that populations are different from one another by likelihood ratio χ2 analysis.
The one exceptional population in which the frequency of insect phenotypes did not match the frequency of plant phenotypes was that at Phillips Tract in Urbana. A possible explanation can be advanced to account for the greater degree of mismatch between plant and insect phenotypes at this site. At all of the other sites, seed samples were taken directly from plants in close proximity to those from which the pupae were collected. To represent the Phillips Tract site, where we have conducted research on this plant/insect interaction for 15 years (5), we used insects collected as pupae from the site for our laboratory colony. Each summer, we collect pupae to renew the culture (no individuals are carried over from the previous year’s culture). The boundaries for this collection are limited to 12 hectares of fields within the research area, whereas the seed collection was confined to roughly 0.3 hectare. Thus, in contrast with the three other sites, the two collections were not closely congruent. If the mismatch obtained at Phillips Tract in fact resulted from differences in plant and insect collection areas, phenotype matching must be an extremely localized phenomenon, even within a seemingly panmictic population.
Although there are several explanations that could account both for differences between populations in phenotype frequencies and for the high degree of phenotype matching between plant and insect, available evidence favors certain hypotheses over others. Large-scale variations in the environment, such as may be expected along a latitudinal transect, could dictate which plant or insect phenotypes are successful in a particular environment. In this scenario, the species that is less constrained by the environment is free to evolve to a stable phenotype frequency suited to the constrained frequency of the species with which it interacts. Insofar as there is no clearly defined clinal variation in phenotype frequencies (Fig. 2), no such environmental constraint is evident in these populations.
Another possibility is that chance events determine the initial gene frequency of one species and that the interacting species is sufficiently flexible to adapt to whatever frequency of phenotypes it encounters. For example, wild parsnips, which occupy disturbed habitats, can be expected to found new populations as disturbance makes new sites available. If the gene frequency of the colonists is not similar to that of the original population, a founder effect can account for variation among populations in wild parsnip phenotypes that may not be correlated with latitudinal variation. A webworm population could subsequently establish itself on this new parsnip population. As it is likely that the webworm adults can travel greater distances than the wind-dispersed parsnip seeds, gene flow could supply the webworm population with ample genetic variation to facilitate the kind of phenotype matching that we observed. The difficulty with this scenario is that the variety of phenotypes is not obviously different among populations; nearly all populations contain the four clusters of phenotypes but simply contain them at different frequencies (Fig. 5). Had we sampled more extensively, we might well have found the rarer clusters in all of the plant and insect populations.
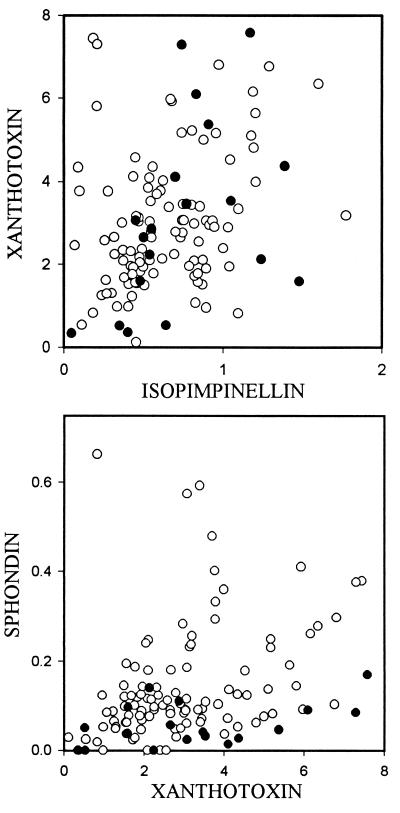
Differences among the populations may result from a form of stable cycling caused by frequency-dependent selection. Shifts in phenotype frequency may occur over time as the plant population evolves to diminish the level of interaction with the insect and the insect evolves to increase the level of interaction. For example, a plant phenotype that has high fitness at one point in time because insect phenotypes capable of exploiting it are rare may experience reduced fitness after it increases in frequency because that increase in frequency has raised the fitness of the rare insect phenotype. Because the possible diversity of phenotypes is limited by genetic variation, this escape/chase interaction cycles endlessly within the universe of possible phenotypes. Although not strictly applicable to our system, which involves multivariate rather than univariate quantitative traits, a model developed by Gavrilets (10) predicts such stable cycling in “exploiter” and “victim” populations. There is reason to believe that stable cycling may occur in these populations. First, with respect to linear furanocoumarins, the range of phenotypes observed in modern populations is not obviously different from what it has been in the past; variation in isopimpinellin and xanthotoxin of herbarium seeds collected as far back as 1873 fall comfortably within the range of modern populations (Fig. 6), and, as we have noted previously, each population appears to contain most or all of the possible variations. Thus, the differences observed among populations may be regarded as spatial variants in the temporal cycling of phenotypes.
Figure 6.
Two-dimensional plots of furanocoumarin concentration (μg/mg) of seeds from herbarium specimens (•) and from the four study populations (○).
Whereas stable cycling may account for variations in linear furanocoumarin content, another possibility exists for the angular furanocoumarin sphondin. Past and present sphondin content does appear to differ; phenotypes with high levels of sphondin not seen in the past are present in modern populations (Fig. 6). One possibility is that the disparity between past and present samples in sphondin content is an artifact of decay of sphondin in the seeds over time. If instability in sphondin is responsible for the pattern, we should see a significant regression between sphondin content and age of sample of our herbarium specimens, yet no significant relationship exists (regression F = 1.828, P = 0.194). An alternative hypothesis is that increases in sphondin content represent an escalation in the classic arms-race analogy. Webworms were first reported to occur in the United States in 1883 (11); the oldest specimen in the Illinois Natural History Survey collection dates back to 1900 (Algonquin, McHenry County, IL). Sphondin previously has been identified as an agent of resistance to webworms (5). In a local population of webworms and parsnips, detectable genetic variation in the ability to metabolize sphondin was absent in webworms, but ample genetic variation in the ability of the plant to produce the compound was found (5, 6). If the absence of genetic variation for sphondin metabolism in webworms is a widespread phenomenon, parsnip populations having genetic variation for production of this compound could in theory produce high-sphondin phenotypes that are more resistant to webworms. These phenotypes would have higher fitness and be favored by natural selection.
This scenario cannot account for the persistence of low-sphondin phenotypes observed in all four populations. Low-sphondin phenotypes may persist in populations because sphondin is a metabolically costly compound to produce; a negative family mean correlation between sphondin concentration and seed production was found in a central Illinois population of parsnips (12). The recent extension of the range of sphondin phenotypes found in the populations surveyed suggests that these phenotypes may include innovations for reducing, but probably not eliminating, the cost of sphondin production.
The high level of phenotype matching observed in interacting populations of parsnip webworms and wild parsnips suggests that coevolutionary processes account for the geographic mosaic that is evident in the differences found among populations (2). Thompson (2) proposed that differences in local successional cycles generate different coevolutionary outcomes in interspecific interactions and such local cycles produce a patchwork, or geographic mosaic, on the landscape scale. Such a patchwork is evidenced by the phenotype matching observed in this interaction. The factors contributing to differences in the local cycles have not yet been identified. Given the “weedy” nature of both insect and plant, restricted for the most part to disturbed habitats, human activities may very well play a role in determining the dynamics of these cycles.
Acknowledgments
We thank Almut Jones for assistance with herbarium specimens, Susanne Timmerman and Jodie Ellis for assistance with dissections for metabolism assays, and Ellen Green, Mark Carroll, and Terry Harrison for collection of pupae and seeds. We thank Curt Lively and Sergey Gavrilets for comments on the manuscript. The research was supported by National Science Foundation Grant DEB 9628977.
References
- 1.Janzen D H. Evolution. 1980;34:611–612. doi: 10.1111/j.1558-5646.1980.tb04849.x. [DOI] [PubMed] [Google Scholar]
- 2.Thompson J N. The Coevolutionary Process. Chicago: Univ. of Chicago Press; 1994. pp. 216–217. [Google Scholar]
- 3.Seger J. Phil Trans R Soc London B. 1988;319:541–555. doi: 10.1098/rstb.1988.0064. [DOI] [PubMed] [Google Scholar]
- 4.Berenbaum M R. In: Herbivores: Their Interactions with Secondary Plant Metabolites. Rosenthal G, Berenbaum M, editors. Vol. 1. New York: Academic; 1991. pp. 221–249. [Google Scholar]
- 5.Berenbaum M R, Zangerl A R, Nitao J K. Evolution. 1986;40:1373–1384. [Google Scholar]
- 6.Berenbaum M R, Zangerl A R. Evolution. 1992;46:1373–1384. doi: 10.1111/j.1558-5646.1992.tb01130.x. [DOI] [PubMed] [Google Scholar]
- 7.Zangerl A R, Berenbaum M R. Ecology. 1993;74:47–54. [Google Scholar]
- 8.Berenbaum M R, Zangerl A R, Nitao J K. Phytochemistry. 1984;23:1809–1810. [Google Scholar]
- 9.Nitao J, Berenbaum M. Ann Entomol Soc Am. 1988;81:485–487. [Google Scholar]
- 10.Gavrilets S. J Theor Biol. 1997;186:527–534. doi: 10.1006/jtbi.1997.0426. [DOI] [PubMed] [Google Scholar]
- 11.Riley C V. Insect Life. 1888;1:94–98. [Google Scholar]
- 12.Zangerl A R, Berenbaum M R. Am Nat. 1997;150:491–504. doi: 10.1086/286077. [DOI] [PubMed] [Google Scholar]