Abstract
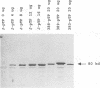
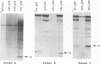
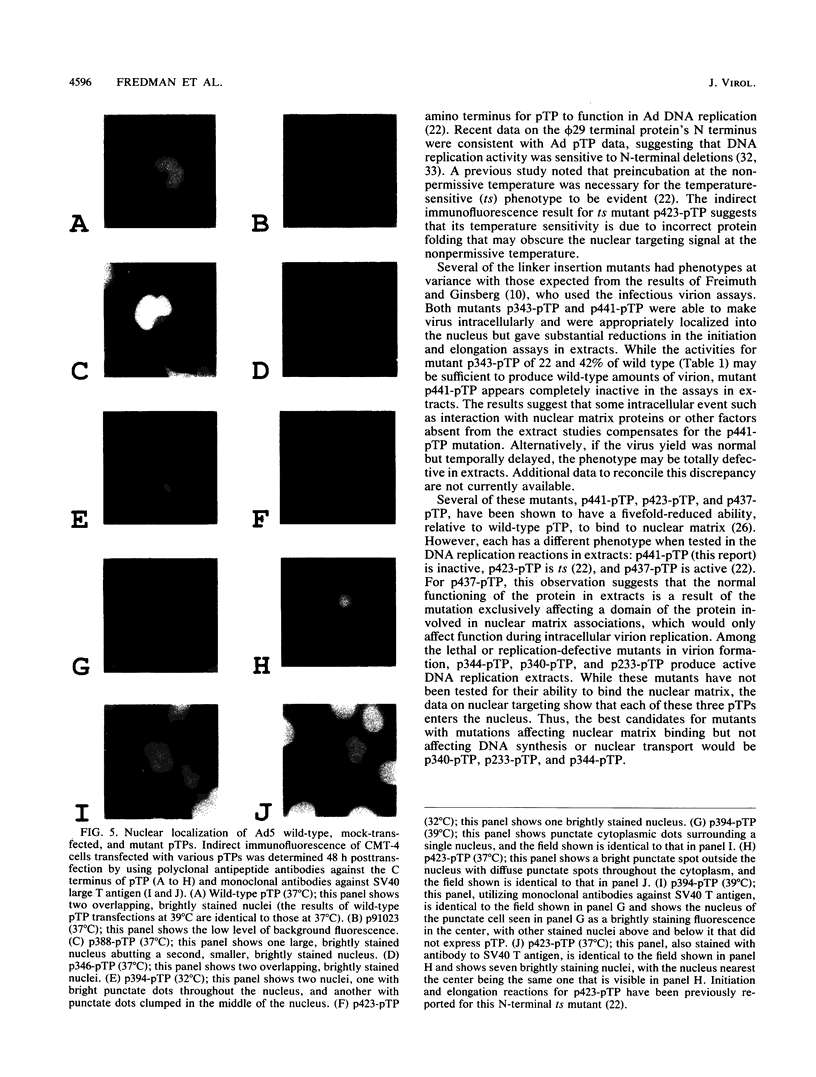
Eighteen linker insertion mutants with mutations in the adenovirus precursor to terminal protein (pTP), which were originally constructed and tested in virions by Freimuth and Ginsberg (Proc. Natl. Acad. Sci. USA 83:7816-7820, 1986), were transferred to expression plasmids for assay of the various functions of the isolated pTP. Function was measured by the ability of individual pTP mutant proteins to participate in the initiation of replication from an adenovirus DNA end, by their activity in assays of DNA elongation, and by the intracellular distribution of pTP demonstrated by indirect immunofluorescence. Ten of the 11 mutants that were active in virion formation were also functional in DNA replication reactions in extracts, while 1 had reduced function. Four mutants with mutations that were lethal to virus production were also inactive in DNA replication reactions. These four mutations are probably located at sites required for the function of pTP in DNA synthesis. Three pTP mutants with mutations that were lethal or partially defective with respect to virion formation were active in reactions requiring pTP for initiation and elongation in extracts. All three of these mutant pTPs targeted normally to the nucleus, suggesting a defect after this step in replication. Since pTP has been reported to bind the nuclear matrix, these pTP mutants may have mutations that define sites necessary for binding to this structure. Several mutants with mutations that lie outside the putative nuclear targeting region were aberrantly localized, suggesting either that additional domains are important in nuclear localization or that there are alterations in protein structure that affect nuclear transport for some pTP mutants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar J. W., Hanson P. I., Polvino-Bodnar M., Zempsky W., Ward D. C. The terminal regions of adenovirus and minute virus of mice DNAs are preferentially associated with the nuclear matrix in infected cells. J Virol. 1989 Oct;63(10):4344–4353. doi: 10.1128/jvi.63.10.4344-4353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Processing of the adenovirus terminal protein. J Virol. 1981 Apr;38(1):272–277. doi: 10.1128/jvi.38.1.272-277.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Mermod N., Horwitz M. S. Protein-protein interactions between adenovirus DNA polymerase and nuclear factor I mediate formation of the DNA replication preinitiation complex. J Biol Chem. 1990 Oct 25;265(30):18634–18642. [PubMed] [Google Scholar]
- Enomoto T., Lichy J. H., Ikeda J. E., Hurwitz J. Adenovirus DNA replication in vitro: purification of the terminal protein in a functional form. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6779–6783. doi: 10.1073/pnas.78.11.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimuth P. I., Ginsberg H. S. Codon insertion mutants of the adenovirus terminal protein. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7816–7820. doi: 10.1073/pnas.83.20.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friefeld B. R., Lichy J. H., Field J., Gronostajski R. M., Guggenheimer R. A., Krevolin M. D., Nagata K., Hurwitz J., Horwitz M. S. The in vitro replication of adenovirus DNA. Curr Top Microbiol Immunol. 1984;110:221–255. doi: 10.1007/978-3-642-46494-2_8. [DOI] [PubMed] [Google Scholar]
- Gerard R. D., Gluzman Y. New host cell system for regulated simian virus 40 DNA replication. Mol Cell Biol. 1985 Nov;5(11):3231–3240. doi: 10.1128/mcb.5.11.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Ariga H. Multiple rounds of adenovirus DNA synthesis in vitro. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1476–1480. doi: 10.1073/pnas.78.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Kenny M. K., Balogh L. A., Hurwitz J. Initiation of adenovirus DNA replication. I. Mechanism of action of a host protein required for replication of adenovirus DNA templates devoid of the terminal protein. J Biol Chem. 1988 Jul 15;263(20):9801–9808. [PubMed] [Google Scholar]
- Kenny M. K., Hurwitz J. Initiation of adenovirus DNA replication. II. Structural requirements using synthetic oligonucleotide adenovirus templates. J Biol Chem. 1988 Jul 15;263(20):9809–9817. [PubMed] [Google Scholar]
- Lichy J. H., Field J., Horwitz M. S., Hurwitz J. Separation of the adenovirus terminal protein precursor from its associated DNA polymerase: role of both proteins in the initiation of adenovirus DNA replication. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5225–5229. doi: 10.1073/pnas.79.17.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill E. A., Kelly T. J. Purification and characterization of nuclear factor III (origin recognition protein C), a sequence-specific DNA binding protein required for efficient initiation of adenovirus DNA replication. J Biol Chem. 1988 Jan 15;263(2):931–937. [PubMed] [Google Scholar]
- Pettit S. C., Horwitz M. S., Engler J. A. Adenovirus preterminal protein synthesized in COS cells from cloned DNA is active in DNA replication in vitro. J Virol. 1988 Feb;62(2):496–500. doi: 10.1128/jvi.62.2.496-500.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit S. C., Horwitz M. S., Engler J. A. Mutations of the precursor to the terminal protein of adenovirus serotypes 2 and 5. J Virol. 1989 Dec;63(12):5244–5250. doi: 10.1128/jvi.63.12.5244-5250.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn G. J., van Miltenburg R. T., Claessens J. A., van der Vliet P. C. Interaction between the octamer-binding protein nuclear factor III and the adenovirus origin of DNA replication. J Virol. 1988 Sep;62(9):3092–3102. doi: 10.1128/jvi.62.9.3092-3102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers D. J., Overman P. F., Chen X. Q., Sussenbach J. S. Linker mutation scanning of the genes encoding the adenovirus type 5 terminal protein precursor and DNA polymerase. Virology. 1991 Jan;180(1):273–284. doi: 10.1016/0042-6822(91)90032-7. [DOI] [PubMed] [Google Scholar]
- Schaack J., Ho W. Y., Freimuth P., Shenk T. Adenovirus terminal protein mediates both nuclear matrix association and efficient transcription of adenovirus DNA. Genes Dev. 1990 Jul;4(7):1197–1208. doi: 10.1101/gad.4.7.1197. [DOI] [PubMed] [Google Scholar]
- Shu L. M., Hong J. S., Wei Y. F., Engler J. A. Nucleotide sequence of the genes encoded in early region 2b of human adenovirus type 12. Gene. 1986;46(2-3):187–195. doi: 10.1016/0378-1119(86)90403-8. [DOI] [PubMed] [Google Scholar]
- Shu L. M., Horwitz M. S., Engler J. A. Expression of enzymatically active adenovirus DNA polymerase from cloned DNA requires sequences upstream of the main open reading frame. Virology. 1987 Dec;161(2):520–526. doi: 10.1016/0042-6822(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Shu L., Pettit S. C., Engler J. A. The precise structure and coding capacity of mRNAs from early region 2B of human adenovirus serotype 2. Virology. 1988 Aug;165(2):348–356. doi: 10.1016/0042-6822(88)90579-x. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Lewis J. B., Chow L. T., Mathews M. B., Smart J. E. Identification of the gene and mRNA for the adenovirus terminal protein precursor. Cell. 1981 Feb;23(2):497–508. doi: 10.1016/0092-8674(81)90145-8. [DOI] [PubMed] [Google Scholar]
- Tremblay M. L., Déry C. V., Talbot B. G., Weber J. In vitro cleavage specificity of the adenovirus type 2 proteinase. Biochim Biophys Acta. 1983 Mar 16;743(2):239–245. doi: 10.1016/0167-4838(83)90220-0. [DOI] [PubMed] [Google Scholar]
- Zaballos A., Lázaro J. M., Méndez E., Mellado R. P., Salas M. Effects of internal deletions on the priming activity of the phage phi 29 terminal protein. Gene. 1989 Nov 30;83(2):187–195. doi: 10.1016/0378-1119(89)90104-2. [DOI] [PubMed] [Google Scholar]
- Zaballos A., Salas M. Functional domains in the bacteriophage phi 29 terminal protein for interaction with the phi 29 DNA polymerase and with DNA. Nucleic Acids Res. 1989 Dec 25;17(24):10353–10366. doi: 10.1093/nar/17.24.10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L. J., Padmanabhan R. Nuclear transport of adenovirus DNA polymerase is facilitated by interaction with preterminal protein. Cell. 1988 Dec 23;55(6):1005–1015. doi: 10.1016/0092-8674(88)90245-0. [DOI] [PubMed] [Google Scholar]