Abstract
The ortho arylation of anilides to form biphenyls via a twofold C–H functionalization/C–C bond-forming process is described. The oxidative coupling takes place in the presence of 5–10 mol % Pd(OAc)2, 10–20 mol % DMSO, and 4–11 equivalents of the aryl substrate in TFA under an oxygen atmosphere. No metal-containing co-catalyst is required.
Organic chemists are faced with the challenges of accessing complex organic molecules efficiently, producing a minimum amount of waste and handling non-renewable or natural resources as economically as possible. In that context, the growing number of reports of catalytic, direct functionalization of C-H bonds by transition metals represents a promising advance.1,2 The application of C-H bond functionalization to the synthesis of biaryls, molecules with important applications in the polymer and pharmaceutical sciences, illustrates the potential utility of these protocols.3
As shown in Scheme 1, biaryl synthesis via C-H bond activation has been achieved via simple4 and, most recently, via twofold5 C-H functionalization approaches. High regioselectivities have generally been observed only when electron-rich arenes5c–e or directing groups (DG)6 such as anilides5a or pyridine5b are used as one of the coupling partners. Despite substantial practical advances, however, important limitations such as the need for Cu or Ag salts as cooxidants (oftentimes in stoichiometric amounts)5a,4d and the requirement of up to a 100 equivalent excess of arene coupling partner limit the synthetic utility of these methods. Fewer examples have been reported of palladium-catalyzed C-H functionalization using molecular oxygen as the only oxidant.7 In one elegant example, Stoltz and coworkers described the oxidative annulation of indoles using a catalytic system of Pd(II)/ligand/O2 (1 atm).8
Scheme 1.
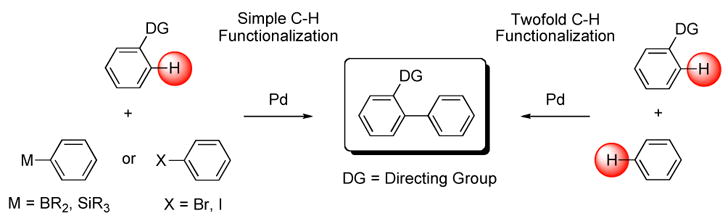
Palladium-catalyzed Simple and Twofold C–H Functionalization in the Presence of a Directing Group
During our investigations in C-H activation-type reactions, we found that a twofold C–H functionalization occurs between anilides and arenes to form substituted biaryls using 5–10 mol % Pd(OAc)2 and 4–11 equivalents of the aryl coupling partner in the presence of oxygen as the terminal oxidant.
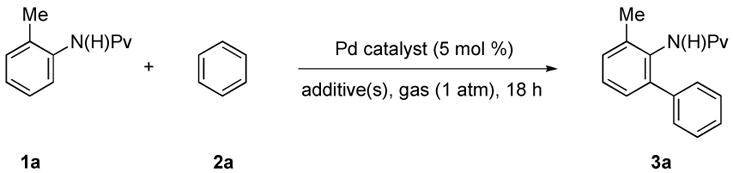
Initially, we studied the coupling of 2-methylpivalanilide (1a) with benzene (2a) in the presence of Pd(OAc)2 and oxygen (1 atm) to afford 3a (Table 1).9 The effects of different palladium sources, additives and temperature were systematically examined. Low conversion of 1a to 3a was observed in presence of 5 mol % Pd(OAc)2 and 11 equivalents (1 mL) of benzene (2a) under an oxygen atmosphere at 80 °C (entry 1). Similar results were obtained when 5 equivalents of acetic acid (AcOH) were added (entry 2). The substitution of trifluoroacetic acid (TFA) for AcOH resulted in better yields, but these results were not fully reproducible. After some experimentation we found that loss of active catalyst through palladium black formation could be slowed by the addition of DMSO (10 mol %), thus affording the expected biaryl in excellent yield (entry 4). The best results were finally obtained at 90 °C, at which temperature 3a was isolated in 92% yield (entry 7).
Table 1.
Screening Results of the Arylation of 2-Methylpivalanilide with Benzene
 | |||||||
|---|---|---|---|---|---|---|---|
| entrya | 2a | Pd catalyst | additive(s) | gas | Temp | convnb | yieldb |
| 1 | 11 equiv | Pd(OAc)2 | - | O2 | 80 °C | 8% | 3% |
| 2 | 11 equiv | Pd(OAc)2 | 5 equiv HOAc | O2 | 80 °C | 11% | 3% |
| 3c | 11 equiv | Pd(OAc)2 | 5 equiv TFA | O2 | 80 °C | 66% | 64% |
| 4 | 11 equiv | Pd(OAc)2 | 5 equiv TFA, 10 mol % DMSO | O2 | 80 °C | 91% | 87% |
| 5 | 11 equiv | Pd(O2CCF3)2 | 5 equiv TFA, 10 mol % DMSO | O2 | 80 °C | 20% | 16% |
| 6 | 11 equiv | PdCl2 | 5 equiv TFA, 10 mol % DMSO | O2 | 80 °C | 8% | - |
| 7 | 11 equiv | Pd(OAc)2 | 5 equiv TFA, 10 mol % DMSO | O2 | 90 °C | 100% | 92%d |
| 8 | 4 equiv | Pd(OAc)2 | 10 equiv TFA, 10 mol % DMSO | O2 | 100 °C | 97% | 78% |
| 9e | 11 equiv | Pd(OAc)2 | 5 equiv TFA, 10 mol % DMSO | air | 80 °C | 82% | 76% |
Reactions carried out on a 1.0 mmol scale.
Corrected GC data with dodecane as internal standard.
Reaction was carried out multiple times with significantly varying results.
Isolated yield: 90% (average of two runs).
Reaction carried out on a 0.2 mmol scale.
The reaction could also be carried out with only 4 equivalents of 2a by increasing the amount of TFA to 10 equivalents, although the corresponding yield was lower. During the course of our work, an elegant related paper by Shi appeared.5a In this disclosure a single example of twofold C-H functionalization using six equivalents of arene coupling partner was reported.10 As shown in entry 9, oxygen could be replaced effectively by air, but lower conversions were generally achieved under these conditions. Poor results were obtained if Pd(OAc)2 was substituted by other palladium salts such as Pd(O2CCF3)2 or PdCl2 (entries 5 and 6).
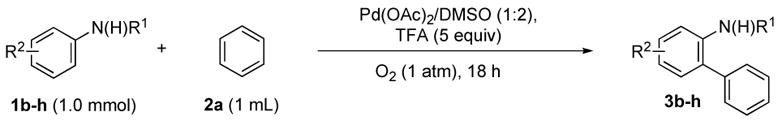
Using these optimized conditions, we next explored the scope and generality of this process using benzene (2a) as an arylating reagent. As shown in Table 2 (entry 1), acetanilides, as well as pivalanilides, can be efficiently arylated using our protocol. Nonetheless, due to their greater stability and selectivity at higher temperatures, we decided to focus our attention on pivalanilides.
Table 2.
Arylation of Anilides with Benzene
 | |||||
|---|---|---|---|---|---|
| entry | anilide | Pd(OAc)2 | temp | biphenyl | yielda |
| 1 |
 (1b) (1b) |
5 mol% | 80 °C |
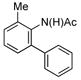
|
70% (3b) |
| 2 |
|
5 mol% | 90 °C |
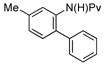
|
91% (3c) |
| 3 |
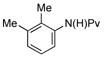 (1d) (1d) |
10 mol% | 90 °C |
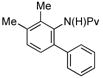
|
84% (3d) |
| 4 |
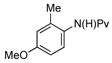 (1e) (1e) |
7.5 mol% | 80 °C |
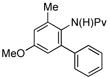
|
87% (3e) |
| 5 |
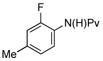 (1f) (1f) |
10 mol% | 90 °C |
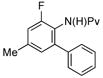
|
86% (3f) |
| 6 |
|
10 mol% | 100 °C |
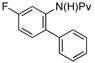
|
68%b (3g) |
| 7 |
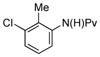 (1h) (1h) |
10 mol% | 55°C |
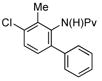
|
59%a,c (3h) |
Isolated yield, average of two runs.
Incomplete conversion of the starting material.
Reaction run for 96 h.
Neutral or electron-rich substituents on the anilide afforded arylated products in good yields of up to 91% (entries 2–5). In the case of the slightly electron-deficient fluorinated compound 1g the temperature had to be raised to 100 °C to obtain a satisfactory yield of 68% (entry 6). However, even with 10 mol % Pd(OAc)2 this reaction could not be driven to completion. Furthermore, the arylated product 3h could be isolated in only 59% yield after 96 h at 55 °C (entry 7). In case of the chlorinated example 1h the reaction had to be carried out at a lower temperature to prevent reduction of the aryl chloride and the immediate formation of palladium black. Electron-deficient substrates such as those substituted by CF3, CO2Me or NO2 groups underwent either no or only trace amounts of arylation.
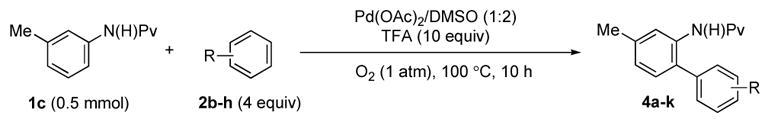
Prompted by these results, we next examined the arylation of anilides with arenes other than benzene (Table 3). Either electron-neutral or electron-rich arenes were equally effective using 7.5–10 mol % Pd(OAc)2. The use of toluene as coupling partner, however, afforded a mixture of ortho, meta and para regioisomers (Table 3, entry 1). The regioselectivity was improved by employing anisole or arenes with several substituents. In particular, the use of veratrole as the arene coupling partner exclusively afforded the arylation product 4d in excellent yield as the only regioisomer.
Table 3.
Arylation of Anilides with Different Aryls.
 | |||||
|---|---|---|---|---|---|
| entry | arene | product | Pd(OAc)2 | selectivitya | yieldb |
| 1 |
|
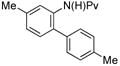 (4a) (4a) |
7.5 mol % | o:m:p 1:16:16 | 82%c |
| 2 |
|
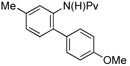 (4b) (4b) |
7.5 mol % | o:m:p 1:2:12 | 77% |
| 3 |
|
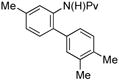 (4c) (4c) |
7.5 mol % | 1,2,3:1,2,4 1:40 | 92% |
| 4 |
|
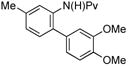 (4d) (4d) |
7.5 mol % | - | 94% |
| 5 |
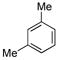
|
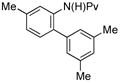 (4e) (4e) |
10 mol % | 1,2,4:1,3,5 1:4.1 | 62%d |
| 6 |
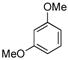
|
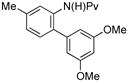 (4f) (4f) |
10 mol % | 1,2,4:1,3,5 1:11 | 79%d |
| 7 |
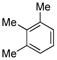
|
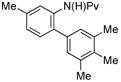 (4g) (4g) |
10 mol % | 1,2,3,4:1,2,3,5 1:10 | 88%e |
| 8 |
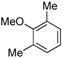
|
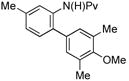 (4h) (4h) |
10 mol % | 1,2,3,4:1,2,3,5 1:20 | 66%d |
The ratio of the regioisomers was detemined by GC.
Isolated yield for the mixture of all regioisomers, average of two runs.
The ratio of the regioisomers was calculated by 1H NMR.
Incomplete conversion.
5 equiv of TFA were used.
Although the electronic effects of the substituents on the arene are considerable in determining the substitution pattern of the product (Table 3, entries 1, 3, 5, 7 vs 2, 4, 6, 8), steric hindrance appears to play a more important role. For example, when 1,3-dimethoxybenzene was used as the arene coupling partner (entry 6), the 1,2,3-regioisomer arising from coupling of the most electron-rich carbon, was not observed. Indeed, with all substrates tested, arylation at less hindered positions was observed almost exclusively. In line with these results, the arylation of 1,4-dimethoxy-benzene was consistently sluggish. These results are in accord with the results recently described by Sanford and coworkers.5b
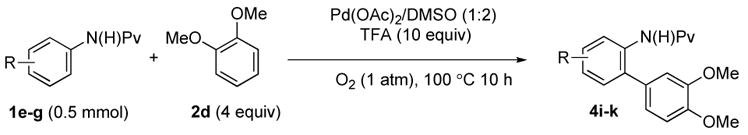
Because of the excellent regioselectivity observed using veratrole 2d as the arene coupling partner, we next turned our attention to the reaction of this substrate with pivalanilides with different substituents (Table 4). As expected, a single regioisomer was observed in all cases, yielding the corresponding biaryls in good yield.
Table 4.
Arylation of Anilides with Veratrole 2d.
 | ||||
|---|---|---|---|---|
| entry | Amide | product | Pd(OAc)2 | yielda |
| 1 |
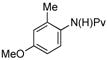 (1e) (1e) |
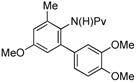 (4i) (4i) |
7.5 mol % | 70% |
| 2 |
 (1f) (1f) |
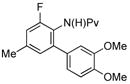 (4j) (4j) |
10 mol % | 70%b |
| 3 |
|
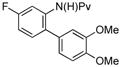 (4k) (4k) |
10 mol % | 73%b |
Isolated yield, average of two runs.
Incomplete conversion.
It is worth mentioning that reactions employing fluorobenzene derivatives, such as 1,3-difluorobenzene or pentafluorobenzene, as the arene coupling partner resulted in notably low conversions under these conditions. The significant acidity of the ortho proton to the fluorine atom make these substrates particulary reactive in a proton abstraction-type mechanism. Thereby, our results suggest that another mechanism may be operative under our reaction conditions.5b,11
In summary, we have developed a practical and mild method for the synthesis of biaryl compounds from simple and readily-accessible anilides and arenes via twofold C-H functionalization using molecular oxygen at atmospheric pressure as the only oxidant. Further mechanistic studies are currently underway.
Supplementary Material
Supporting Information Available: Experimental procedures and spectral data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank the National Institutes of Health (GM46059) for support of this work. G. B. and J. G.-F. thank the German Academic Exchange Service (DAAD) and the Spanish M.E.C. for postdoctoral fellowships. BASF is acknowledged for a generous gift of Pd(OAc)2. We also thank Amgen, Merck, and Boehringer Ingelheim for additional unrestricted funds. The Varian NMR instruments used for this publication were purchased with funds from the NSF (CHE 9808061 and DBI 9729592).
References
- 1.For recent reviews of C–H functionalization, see: Alberico D, Scott ME, Lautens M. Chem Rev. 2007;107:174. doi: 10.1021/cr0509760.Godula K, Sames D. Science. 2006;312:67. doi: 10.1126/science.1114731.Kakiuchi F, Chatani N. Adv Synth Catal. 2003;345:1077.Ritleng V, Sirlin C, Pfeffer M. Chem Rev. 2002;102:1731. doi: 10.1021/cr0104330.
- 2.For selected examples using palladium as a catalyst, see: Campeau LC, Schipper DJ, Fagnou K. J Am Chem Soc. 2008;130:3276. doi: 10.1021/ja710451s.Inamoto K, Saito T, Katsuno M, Sakamoto T, Hiroya K. Org Lett. 2007;9:2931. doi: 10.1021/ol0711117.Cai G, Fu Y, Le Y, Wan X, Shi Z. J Am Chem Soc. 2007;129:7666. doi: 10.1021/ja070588a.Delcamp JH, White MC. J Am Chem Soc. 2006;128:15076. doi: 10.1021/ja066563d.Wan X, Ma Z, Li B, Zhang K, Cao S, Zhang S, Shi Z. J Am Chem Soc. 2006;128:7416. doi: 10.1021/ja060232j.Grimster NP, Gauntlett C, Godfrey CRA, Gaunt MJ. Angew Chem Int Ed. 2005;44:3125. doi: 10.1002/anie.200500468.Tsang WCP, Zheng N, Buchwald SL. J Am Chem Soc. 2005;127:14560. doi: 10.1021/ja055353i.Boele MDK, van Strijdonck GPF, de Vries AHM, Kamer PCJ, de Vries JG, van Leeuwen PWNM. J Am Chem Soc. 2002;124:1586. doi: 10.1021/ja0176907.
- 3.Bringmann G, Günther C, Ochse M, Schupp O, Tasler S. In: Progress in the Chemistry of Organic Natural Products. Herz W, Falk H, Kirby GW, Moore RE, editors. Vol. 82. Springer; New York: 2001. pp. 1–293. [DOI] [PubMed] [Google Scholar]
- 4.(a) Shi Z, Li B, Wan X, Cheng J, Fang Z, Cao B, Qin C, Wang Y. Angew Chem Int Ed. 2007;46:5554. doi: 10.1002/anie.200700590. [DOI] [PubMed] [Google Scholar]; (b) Yang S, Li B, Wan X, Shi Z. J Am Chem Soc. 2007;129:6066. doi: 10.1021/ja070767s. [DOI] [PubMed] [Google Scholar]; (c) Chiong HA, Pham QN, Daugulis O. J Am Chem Soc. 2007;129:9879. doi: 10.1021/ja071845e. [DOI] [PubMed] [Google Scholar]; (d) Daugulis O, Zaitsev VG. Angew Chem Int Ed. 2005;44:4046. doi: 10.1002/anie.200500589. [DOI] [PubMed] [Google Scholar]
- 5.(a) Li BJ, Tian SL, Fang Z, Shi ZJ. Angew Chem Int Ed. 2008;47:1115. doi: 10.1002/anie.200704092. [DOI] [PubMed] [Google Scholar]; (b) Hull KL, Sanford MS. J Am Chem Soc. 2007;129:11904. doi: 10.1021/ja074395z. [DOI] [PubMed] [Google Scholar]; (c) Stuart DR, Villemure E, Fagnou K. J Am Chem Soc. 2007;129:12072. doi: 10.1021/ja0745862. [DOI] [PubMed] [Google Scholar]; (d) Stuart DR, Fagnou K. Science. 2007;316:1172. doi: 10.1126/science.1141956. [DOI] [PubMed] [Google Scholar]; (e) Dwight TA, Rue NR, Charyk D, Josselyn R, DeBoef B. Org Lett. 2007;9:3137. doi: 10.1021/ol071308z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Yu JQ, Giri R, Chen X. Org Biomol Chem. 2006;4:4041. doi: 10.1039/b611094k. [DOI] [PubMed] [Google Scholar]; (b) Daugulis O, Zaitsev VG, Shabashov D, Pham QN, Lazareva A. Synlett. 2006:3382. [Google Scholar]
- 7.(a) Beck EM, Grimster NP, Hatley R, Gaunt MJ. J Am Chem Soc. 2006;128:2528. doi: 10.1021/ja058141u. [DOI] [PubMed] [Google Scholar]; (b) Stahl S. Angew Chem Int Ed. 2004;43:3400. doi: 10.1002/anie.200300630. [DOI] [PubMed] [Google Scholar]; (b) Dams M, De Vos DE, Celen S, Jacobs PA. Angew Chem Int Ed. 2003;42:3512. doi: 10.1002/anie.200351524. [DOI] [PubMed] [Google Scholar]; (c) Hagelin H, Oslob JD, Åkermark B Chem-Eur J. 1999;5:2413. [Google Scholar]; (d) Shue RS. J Chem Soc Chem Commun. 1971:1510. [Google Scholar]
- 8.(a) Ferreira EM, Stoltz BM. J Am Chem Soc. 2003;125:9578. doi: 10.1021/ja035054y. [DOI] [PubMed] [Google Scholar]; (b) Ferreira EM, Zhang H, Stoltz BM. Tetrahedron. 2008 doi: 10.1016/j.tet.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unsubstituted pivalanilide gave rise to a variable mixture of mono- and bis-ortho arylated products. Similar results were observed by Daugulis et al. See reference 4d for details.
- 10.According to the Supporting Information of reference 5a, most of the examples use 28–37 equivalents of arene counterpart.
- 11.(a) Lafrance M, Rowley CN, Woo TK, Fagnou K. J Am Chem Soc. 2006;128:8754. doi: 10.1021/ja062509l. [DOI] [PubMed] [Google Scholar]; (b) Garcia-Cuadrado D, Braga AAC, Maseras F, Echavarren AM. J Am Chem Soc. 2006;128:1066. doi: 10.1021/ja056165v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available: Experimental procedures and spectral data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.


