Abstract
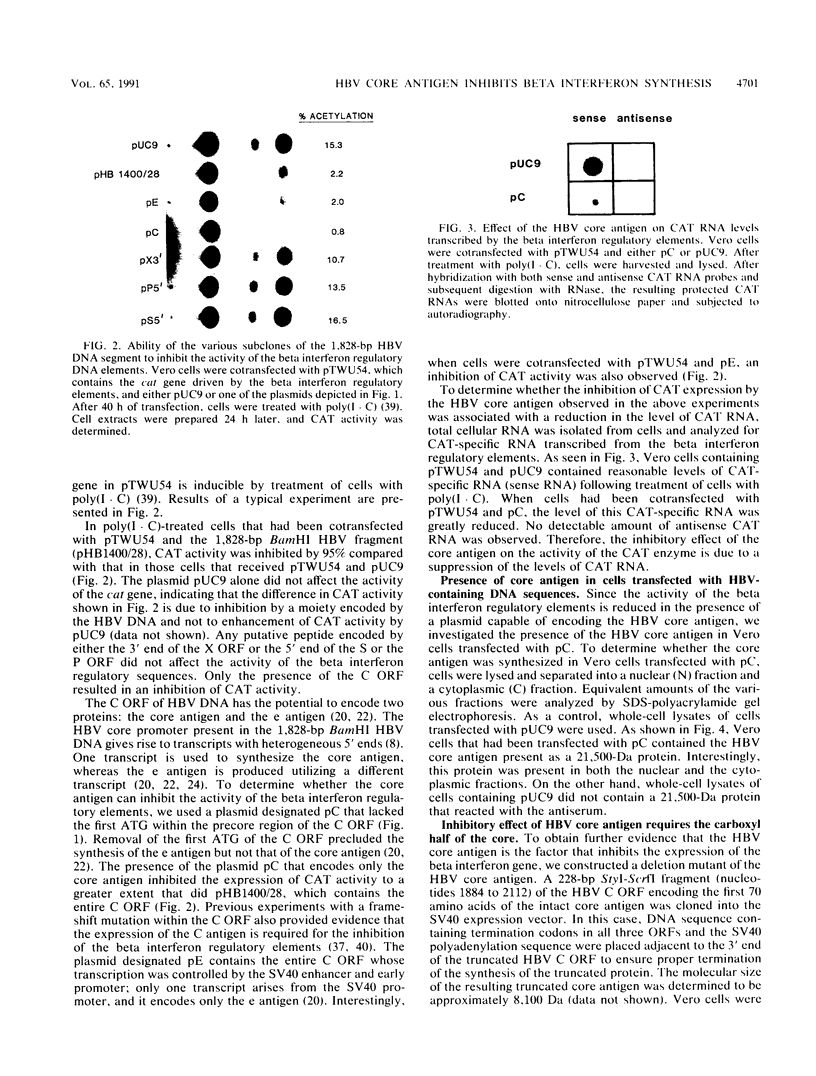
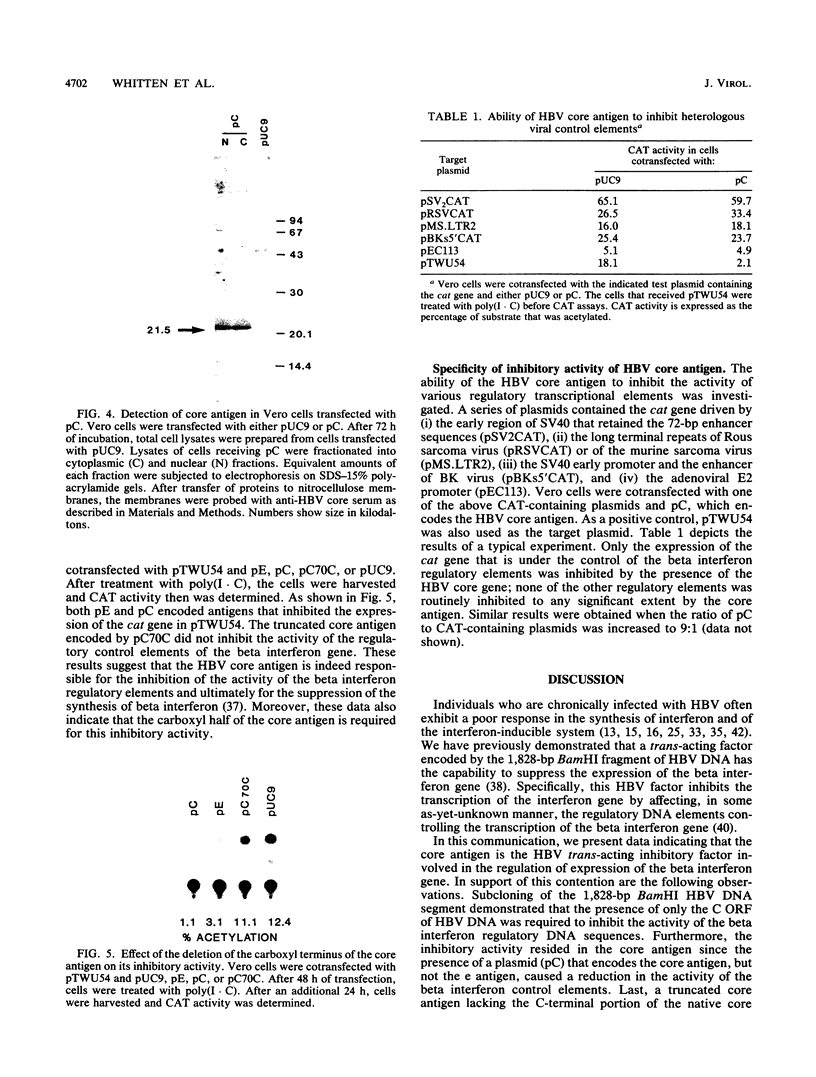
The identity of the trans-acting factor encoded by the 1,828-bp BamHI DNA fragment of hepatitis B virus (HBV) that suppresses the transcription of the human beta interferon gene was investigated. Each complete and partial open reading frame (ORF) present within the 1,828-bp BamHI HBV DNA fragment was cloned into a simian virus 40 expression vector, and the resulting gene products were assayed for their ability to inhibit the activity of the regulatory DNA region that governs the expression of the beta interferon gene. Only the proteins encoded by the C ORF inhibited the activity of the beta interferon regulatory DNA region; putative proteins encoded by the partial X, P, and S ORFs present in the 1,828-bp BamHI HBV DNA fragment had no effect. A plasmid encoding only the native HBV core antigen, but not one coding for a truncated core antigen, possessed this inhibitory activity. The inhibition by the core antigen was specific for the regulatory elements of the beta interferon gene; none of a variety of viral transcriptional elements was inhibited.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartenschlager R., Schaller H. The amino-terminal domain of the hepadnaviral P-gene encodes the terminal protein (genome-linked protein) believed to prime reverse transcription. EMBO J. 1988 Dec 20;7(13):4185–4192. doi: 10.1002/j.1460-2075.1988.tb03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavand M. R., Laub O. Two proteins with reverse transcriptase activities associated with hepatitis B virus-like particles. J Virol. 1988 Feb;62(2):626–628. doi: 10.1128/jvi.62.2.626-628.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay P., Mandart E., Hampe A., Fitoussi F., Tiollais P., Galibert F. Localization on the viral genome and nucleotide sequence of the gene coding for the two major polypeptides of the hepatitis B surface antigen (HBs Ag). Nucleic Acids Res. 1979 Sep 25;7(2):335–346. doi: 10.1093/nar/7.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings I. W., Browne J. K., Salser W. A., Tyler G. V., Snyder R. L., Smolec J. M., Summers J. Isolation, characterization, and comparison of recombinant DNAs derived from genomes of human hepatitis B virus and woodchuck hepatitis virus. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1842–1846. doi: 10.1073/pnas.77.4.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. L., Hoofnagle J. H. Interferon in viral hepatitis: role in pathogenesis and treatment. Hepatology. 1986 Sep-Oct;6(5):1038–1041. doi: 10.1002/hep.1840060537. [DOI] [PubMed] [Google Scholar]
- Eckhardt S. G., Milich D. R., McLachlan A. Hepatitis B virus core antigen has two nuclear localization sequences in the arginine-rich carboxyl terminus. J Virol. 1991 Feb;65(2):575–582. doi: 10.1128/jvi.65.2.575-582.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders G. H., Ganem D., Varmus H. Mapping the major transcripts of ground squirrel hepatitis virus: the presumptive template for reverse transcriptase is terminally redundant. Cell. 1985 Aug;42(1):297–308. doi: 10.1016/s0092-8674(85)80125-2. [DOI] [PubMed] [Google Scholar]
- Gallina A., Bonelli F., Zentilin L., Rindi G., Muttini M., Milanesi G. A recombinant hepatitis B core antigen polypeptide with the protamine-like domain deleted self-assembles into capsid particles but fails to bind nucleic acids. J Virol. 1989 Nov;63(11):4645–4652. doi: 10.1128/jvi.63.11.4645-4652.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. A., Walsh J. H., Purcell R. H. Failure to demonstrate circulating interferon during incubation period and acute stage of transfusion-associated hepatitis. Proc Soc Exp Biol Med. 1971 Mar;136(3):853–856. doi: 10.3181/00379727-136-35379. [DOI] [PubMed] [Google Scholar]
- Hirsch R. C., Lavine J. E., Chang L. J., Varmus H. E., Ganem D. Polymerase gene products of hepatitis B viruses are required for genomic RNA packaging as wel as for reverse transcription. Nature. 1990 Apr 5;344(6266):552–555. doi: 10.1038/344552a0. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Lever A. M., Thomas H. C. Evidence for a deficiency of interferon production in patients with chronic hepatitis B virus infection acquired in adult life. Hepatology. 1986 Sep-Oct;6(5):962–965. doi: 10.1002/hep.1840060525. [DOI] [PubMed] [Google Scholar]
- Kinoshita R., Miura K., Suzuki S., Uchino H. Lack of correlation between interferon production of mononuclear cells and virus replication in chronic hepatitis B virus infection. J Clin Microbiol. 1979 Dec;10(6):923–925. doi: 10.1128/jcm.10.6.923-925.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Gruss P., Pozzatti R., Khoury G. Characterization of enhancer elements in the long terminal repeat of Moloney murine sarcoma virus. J Virol. 1984 Jan;49(1):183–189. doi: 10.1128/jvi.49.1.183-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeken M. R., Khoury G., Brady J. Stimulation of the adenovirus E2 promoter by simian virus 40 T antigen or E1A occurs by different mechanisms. Mol Cell Biol. 1986 Jun;6(6):2020–2026. doi: 10.1128/mcb.6.6.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A., Milich D. R., Raney A. K., Riggs M. G., Hughes J. L., Sorge J., Chisari F. V. Expression of hepatitis B virus surface and core antigens: influences of pre-S and precore sequences. J Virol. 1987 Mar;61(3):683–692. doi: 10.1128/jvi.61.3.683-692.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano L., Miescher G. C., Goodall A. H., Wiedmann K. H., Janossy G., Thomas H. C. Hepatitis B virus and HLA antigen display in the liver during chronic hepatitis B virus infection. Hepatology. 1982 Sep-Oct;2(5):557–561. doi: 10.1002/hep.1840020508. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Laub O., Rutter W. J. Hepatitis B virus gene function: the precore region targets the core antigen to cellular membranes and causes the secretion of the e antigen. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1578–1582. doi: 10.1073/pnas.83.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Yeh C. T., Yen T. S. Transport of hepatitis B virus precore protein into the nucleus after cleavage of its signal peptide. J Virol. 1989 Dec;63(12):5238–5243. doi: 10.1128/jvi.63.12.5238-5243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitrine A., Chousterman S., Chousterman M., Naveau S., Thang M. N., Chaput J. C. Lack of in vivo activation of the interferon system in HBsAg-positive chronic active hepatitis. Hepatology. 1985 Mar-Apr;5(2):171–174. doi: 10.1002/hep.1840050202. [DOI] [PubMed] [Google Scholar]
- Ponzetto A., Cote P. J., Ford E. C., Purcell R. H., Gerin J. L. Core antigen and antibody in woodchucks after infection with woodchuck hepatitis virus. J Virol. 1984 Oct;52(1):70–76. doi: 10.1128/jvi.52.1.70-76.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziwill G., Tucker W., Schaller H. Mutational analysis of the hepatitis B virus P gene product: domain structure and RNase H activity. J Virol. 1990 Feb;64(2):613–620. doi: 10.1128/jvi.64.2.613-620.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal N., Kress M., Gruss P., Khoury G. BK viral enhancer element and a human cellular homolog. Science. 1983 Nov 18;222(4625):749–755. doi: 10.1126/science.6314501. [DOI] [PubMed] [Google Scholar]
- Seto E., Yen T. S., Peterlin B. M., Ou J. H. Trans-activation of the human immunodeficiency virus long terminal repeat by the hepatitis B virus X protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8286–8290. doi: 10.1073/pnas.85.21.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E., Zhou D. X., Peterlin B. M., Yen T. S. trans-activation by the hepatitis B virus X protein shows cell-type specificity. Virology. 1989 Dec;173(2):764–766. doi: 10.1016/0042-6822(89)90594-1. [DOI] [PubMed] [Google Scholar]
- Siddiqui A., Gaynor R., Srinivasan A., Mapoles J., Farr R. W. trans-activation of viral enhancers including long terminal repeat of the human immunodeficiency virus by the hepatitis B virus X protein. Virology. 1989 Apr;169(2):479–484. doi: 10.1016/0042-6822(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Spandau D. F., Lee C. H. trans-activation of viral enhancers by the hepatitis B virus X protein. J Virol. 1988 Feb;62(2):427–434. doi: 10.1128/jvi.62.2.427-434.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring D. N., Ou J. H., Masiarz F. R., Rutter W. J. A signal peptide encoded within the precore region of hepatitis B virus directs the secretion of a heterogeneous population of e antigens in Xenopus oocytes. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8405–8409. doi: 10.1073/pnas.85.22.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiehm E. R., Kronenberg L. H., Rosenblatt H. M., Bryson Y., Merigan T. C. UCLA conference. Interferon: immunobiology and clinical significance. Ann Intern Med. 1982 Jan;96(1):80–93. doi: 10.7326/0003-4819-96-1-80. [DOI] [PubMed] [Google Scholar]
- Taylor P. E., Zuckerman A. J. Non-production of interfering substances by serum from patients with infectious hepatitis. J Med Microbiol. 1968 Nov;1(2):217–219. doi: 10.1099/00222615-1-2-217. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Tolentino P., Dianzani F., Zucca M., Giacchino R. Decreased interferon response by lymphocytes from children with chronic hepatitis. J Infect Dis. 1975 Oct;132(4):459–461. doi: 10.1093/infdis/132.4.459. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Lee C. H., Lin P. M., Schloemer R. H. Hepatitis B virus suppresses expression of human beta-interferon. Proc Natl Acad Sci U S A. 1988 Jan;85(1):252–256. doi: 10.1073/pnas.85.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Robinson W. S. Hepatitis B virus X gene can transactivate heterologous viral sequences. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2046–2050. doi: 10.1073/pnas.86.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Schloemer R. H. Transcription of the human beta interferon gene is inhibited by hepatitis B virus. J Virol. 1989 Jul;63(7):3065–3071. doi: 10.1128/jvi.63.7.3065-3071.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Schloemer R. H. Transcriptional trans-activating function of hepatitis B virus. J Virol. 1987 Nov;61(11):3448–3453. doi: 10.1128/jvi.61.11.3448-3453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Hallenbeck L. A. Effect of virus infections on target cell susceptibility to natural killer cell-mediated lysis. J Immunol. 1980 May;124(5):2491–2497. [PubMed] [Google Scholar]
- Wheelock E. F., Schenker S., Combes B. Absence of circulating interferon in patients with infectious and serum hepatitis. Proc Soc Exp Biol Med. 1968 May;128(1):251–253. doi: 10.3181/00379727-128-32989. [DOI] [PubMed] [Google Scholar]