Abstract
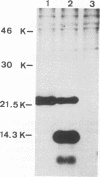
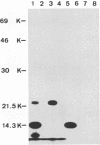
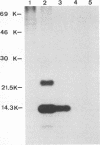
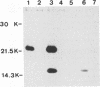
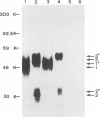
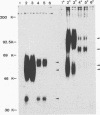
Virions from hog cholera virus (HCV), a member of the genus Pestivirus, were analyzed by using specific antibodies. The nucleocapsid protein was found to be a 14-kDa molecule (HCV p14). An equivalent protein could also be demonstrated for virions from another pestivirus, bovine viral diarrhea virus. The HCV envelope is composed of three glycoproteins, HCV gp44/48, gp33, and gp55. All three exist in the form of disulfide-linked dimers in virus-infected cells and in virions; HCV gp44/48 and gp55 each form homodimers, whereas gp55 is also found dimerized with gp33. Such complex covalent interactions between structural glycoproteins have not been described so far for any RNA virus.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boege U., Wengler G., Wengler G., Wittmann-Liebold B. Primary structures of the core proteins of the alphaviruses Semliki Forest virus and Sindbis virus. Virology. 1981 Aug;113(1):293–303. doi: 10.1016/0042-6822(81)90156-2. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Hahn C. S., Galler R., Rice C. M. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- Colett M. S., Larson R., Gold C., Strick D., Anderson D. K., Purchio A. F. Molecular cloning and nucleotide sequence of the pestivirus bovine viral diarrhea virus. Virology. 1988 Jul;165(1):191–199. doi: 10.1016/0042-6822(88)90672-1. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Anderson D. K., Retzel E. Comparisons of the pestivirus bovine viral diarrhoea virus with members of the flaviviridae. J Gen Virol. 1988 Oct;69(Pt 10):2637–2643. doi: 10.1099/0022-1317-69-10-2637. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Larson R., Belzer S. K., Retzel E. Proteins encoded by bovine viral diarrhea virus: the genomic organization of a pestivirus. Virology. 1988 Jul;165(1):200–208. doi: 10.1016/0042-6822(88)90673-3. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Moennig V., Horzinek M. C. Recent advances in pestivirus research. J Gen Virol. 1989 Feb;70(Pt 2):253–266. doi: 10.1099/0022-1317-70-2-253. [DOI] [PubMed] [Google Scholar]
- Donis R. O., Corapi W., Dubovi E. J. Neutralizing monoclonal antibodies to bovine viral diarrhoea virus bind to the 56K to 58K glycoprotein. J Gen Virol. 1988 Jan;69(Pt 1):77–86. doi: 10.1099/0022-1317-69-1-77. [DOI] [PubMed] [Google Scholar]
- Enzmann P. J. In vivo labelling of viral proteins with 75selenomethionine. J Virol Methods. 1982 Nov;5(3-4):243–246. doi: 10.1016/0166-0934(82)90014-3. [DOI] [PubMed] [Google Scholar]
- Goding J. W., Harris A. W. Subunit structure of cell surface proteins: disulfide bonding in antigen receptors, Ly-2/3 antigens, and transferrin receptors of murine T and B lymphocytes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4530–4534. doi: 10.1073/pnas.78.7.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Use of protein A-bearing staphylococci for the immunoprecipitation and isolation of antigens from cells. Methods Enzymol. 1981;73(Pt B):442–459. doi: 10.1016/0076-6879(81)73084-2. [DOI] [PubMed] [Google Scholar]
- Laude H. Hog cholera virus: art and facts. Ann Rech Vet. 1987;18(2):127–138. [PubMed] [Google Scholar]
- Matthaeus W. Detection of three polypeptides in preparations of bovine viral diarrhoea virus. Arch Virol. 1979;59(4):299–305. doi: 10.1007/BF01317470. [DOI] [PubMed] [Google Scholar]
- Meyers G., Rümenapf T., Thiel H. J. Molecular cloning and nucleotide sequence of the genome of hog cholera virus. Virology. 1989 Aug;171(2):555–567. doi: 10.1016/0042-6822(89)90625-9. [DOI] [PubMed] [Google Scholar]
- Moormann R. J., Warmerdam P. A., van der Meer B., Schaaper W. M., Wensvoort G., Hulst M. M. Molecular cloning and nucleotide sequence of hog cholera virus strain Brescia and mapping of the genomic region encoding envelope protein E1. Virology. 1990 Jul;177(1):184–198. doi: 10.1016/0042-6822(90)90472-4. [DOI] [PubMed] [Google Scholar]
- Nowak T., Färber P. M., Wengler G., Wengler G. Analyses of the terminal sequences of West Nile virus structural proteins and of the in vitro translation of these proteins allow the proposal of a complete scheme of the proteolytic cleavages involved in their synthesis. Virology. 1989 Apr;169(2):365–376. doi: 10.1016/0042-6822(89)90162-1. [DOI] [PubMed] [Google Scholar]
- Nowak T., Wengler G. Analysis of disulfides present in the membrane proteins of the West Nile flavivirus. Virology. 1987 Jan;156(1):127–137. doi: 10.1016/0042-6822(87)90443-0. [DOI] [PubMed] [Google Scholar]
- Pritchett R. F., Zee Y. C. Structural proteins of bovine viral diarrhea virus. Am J Vet Res. 1975 Dec;36(12):1731–1734. [PubMed] [Google Scholar]
- Purchio A. F., Larson R., Collett M. S. Characterization of bovine viral diarrhea virus proteins. J Virol. 1984 May;50(2):666–669. doi: 10.1128/jvi.50.2.666-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Linares A., Cahour A., Després P., Girard M., Bouloy M. Processing of yellow fever virus polyprotein: role of cellular proteases in maturation of the structural proteins. J Virol. 1989 Oct;63(10):4199–4209. doi: 10.1128/jvi.63.10.4199-4209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rümenapf T., Meyers G., Stark R., Thiel H. J. Hog cholera virus--characterization of specific antiserum and identification of cDNA clones. Virology. 1989 Jul;171(1):18–27. doi: 10.1016/0042-6822(89)90506-0. [DOI] [PubMed] [Google Scholar]
- Rümenapf T., Stark R., Meyers G., Thiel H. J. Structural proteins of hog cholera virus expressed by vaccinia virus: further characterization and induction of protective immunity. J Virol. 1991 Feb;65(2):589–597. doi: 10.1128/jvi.65.2.589-597.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R., Rümenapf T., Meyers G., Thiel H. J. Genomic localization of hog cholera virus glycoproteins. Virology. 1990 Jan;174(1):286–289. doi: 10.1016/0042-6822(90)90076-4. [DOI] [PubMed] [Google Scholar]
- Strandström H., Veijalainen P., Moennig V., Hunsmann G., Schwarz H., Schäfer W. C-type particles produced by a permanent cell line from a leukemic pig. I. Origin and properties of the host cells and some evidence for the occurrence of C-type-like particles. Virology. 1974 Jan;57(1):175–178. doi: 10.1016/0042-6822(74)90118-4. [DOI] [PubMed] [Google Scholar]
- Strebel K., Beck E., Strohmaier K., Schaller H. Characterization of foot-and-mouth disease virus gene products with antisera against bacterially synthesized fusion proteins. J Virol. 1986 Mar;57(3):983–991. doi: 10.1128/jvi.57.3.983-991.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland E., Stark R., Haas B., Rümenapf T., Meyers G., Thiel H. J. Pestivirus glycoprotein which induces neutralizing antibodies forms part of a disulfide-linked heterodimer. J Virol. 1990 Aug;64(8):3563–3569. doi: 10.1128/jvi.64.8.3563-3569.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensvoort G., Boonstra J., Bodzinga B. G. Immunoaffinity purification and characterization of the envelope protein E1 of hog cholera virus. J Gen Virol. 1990 Mar;71(Pt 3):531–540. doi: 10.1099/0022-1317-71-3-531. [DOI] [PubMed] [Google Scholar]
- Westaway E. G., Brinton M. A., Gaidamovich SYa, Horzinek M. C., Igarashi A., Käriäinen L., Lvov D. K., Porterfield J. S., Russell P. K., Trent D. W. Togaviridae. Intervirology. 1985;24(3):125–139. doi: 10.1159/000149632. [DOI] [PubMed] [Google Scholar]