Abstract
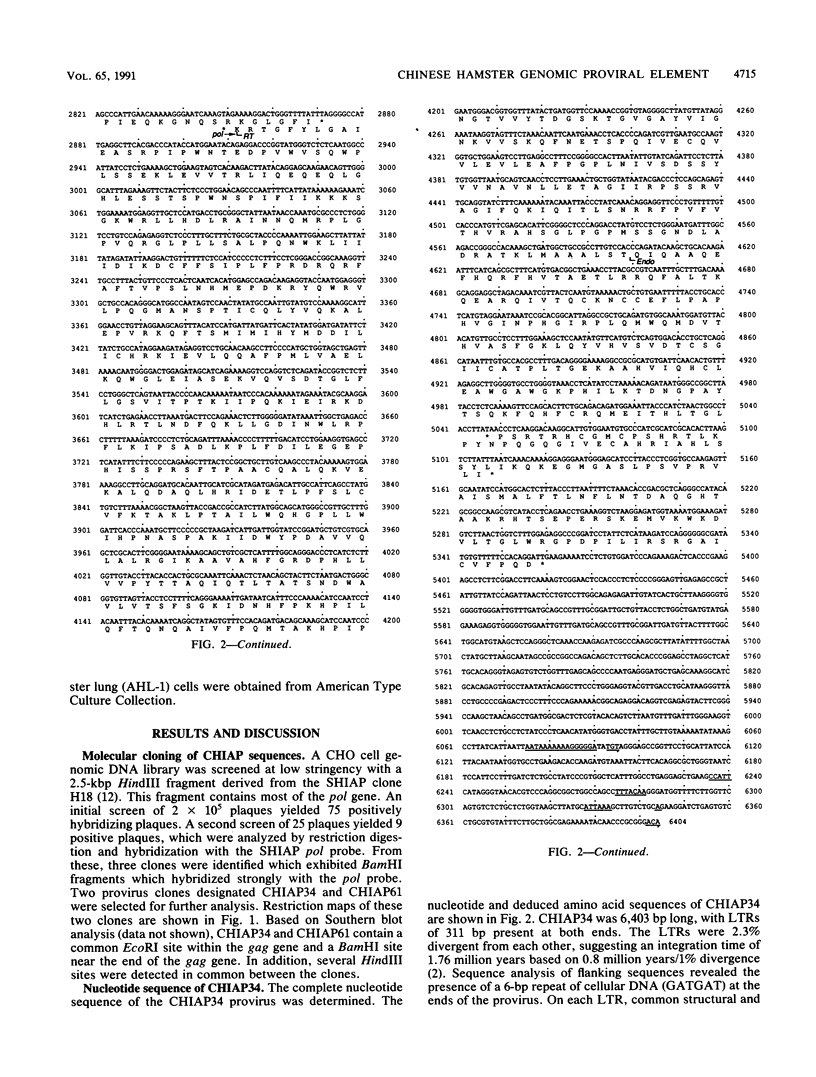
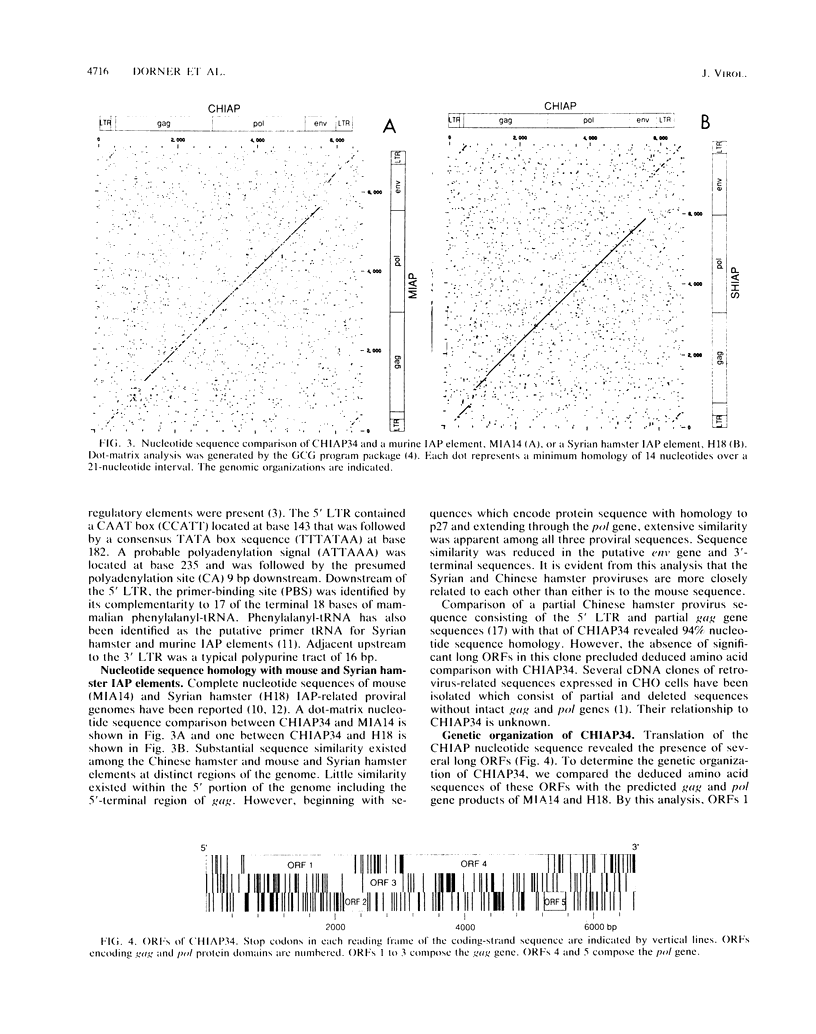
We report here the nucleotide sequence of a full-length Chinese hamster genomic proviral element, CHIAP34. CHIAP34 is 6,403 bp long with long terminal repeats of 311 bp at each end. The genetic organization of CHIAP34 was determined by comparison with intracisternal A particle (IAP) genetic elements from the mouse and Syrian hamster. Extensive homology at the nucleotide and deduced amino acid sequence levels was observed between CHIAP34 and the mouse and Syrian hamster IAP elements. CHIAP34 may represent a defective Chinese hamster IAP genetic element. The gag gene consists of 837 codons, of which 558 codons are in a single long open reading frame followed by several frameshifts. The pol gene begins with a -1 frameshift and consists of a long open reading frame of 753 codons followed by a short open reading frame of 103 codons. The putative env region contains multiple termination codons in all reading frames. CHIAP34 is representative of the predominant retroviral elements in the Chinese hamster ovary cell genome present at around 80 copies per haploid genome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. P., Lie Y. S., Low M. A., Williams S. R., Fennie E. H., Nguyen T. P., Wurm F. M. Presence and transcription of intracisternal A-particle-related sequences in CHO cells. J Virol. 1990 May;64(5):2021–2032. doi: 10.1128/jvi.64.5.2021-2032.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Callahan R., Sherr C. J., Chapman V., Todaro G. J. Two distinct endogenous type C viruses isolated from the asian rodent Mus cervicolor: conservation of virogene sequences in related rodent species. J Virol. 1977 Mar;21(3):849–862. doi: 10.1128/jvi.21.3.849-862.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine U. I., Margulies I., Demsey A. E., Suskind R. G. Quantitative electron microscopy of intracytoplasmic type A particles at kinetochores of metaphase chromosomes isolated from Chinese hamster and murine cell lines. J Gen Virol. 1979 Dec;45(3):631–640. doi: 10.1099/0022-1317-45-3-631. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Lesser J., Lasneret J., Canivet M., Emanoil-Ravier R., Périès J. Simultaneous activation by 5-azacytidine of intracisternal R particles and murine intracisternal-A particle related sequences in Syrian hamster cells. Virology. 1986 Nov;155(1):249–256. doi: 10.1016/0042-6822(86)90184-4. [DOI] [PubMed] [Google Scholar]
- Lieber M. M., Benveniste R. E., Livingston D. M., Todaro G. J. Mammalian cells in culture frequently release type C viruses. Science. 1973 Oct 5;182(4107):56–59. doi: 10.1126/science.182.4107.56. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Comparison of the sequence organization of related retrovirus-like multigene families in three evolutionarily distant rodent genomes. Nucleic Acids Res. 1983 Jul 11;11(13):4391–4408. doi: 10.1093/nar/11.13.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietz J. A., Grossman Z., Lueders K. K., Kuff E. L. Nucleotide sequence of a complete mouse intracisternal A-particle genome: relationship to known aspects of particle assembly and function. J Virol. 1987 Oct;61(10):3020–3029. doi: 10.1128/jvi.61.10.3020-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Ohishi H. Long terminal repeat sequences of intracisternal A particle genes in the Syrian hamster genome: identification of tRNAPhe as a putative primer tRNA. Nucleic Acids Res. 1983 Oct 25;11(20):7169–7179. doi: 10.1093/nar/11.20.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Toh H., Miyata T., Awaya T. Nucleotide sequence of the Syrian hamster intracisternal A-particle gene: close evolutionary relationship of type A particle gene to types B and D oncovirus genes. J Virol. 1985 Aug;55(2):387–394. doi: 10.1128/jvi.55.2.387-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servenay M., Kupiec J. J., d'Auriol L., Galibert F., Peries J., Emanoil-Ravier R. Nucleotide sequence of the Chinese hamster intracisternal A-particle genomic region corresponding to 5'LTR-GAG. Nucleic Acids Res. 1988 Aug 11;16(15):7725–7725. doi: 10.1093/nar/16.15.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. C., Kobori J. A., Siu G., Hood L. E. Specific-primer-directed DNA sequencing. Anal Biochem. 1986 Apr;154(1):353–360. doi: 10.1016/0003-2697(86)90536-1. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]




