Abstract
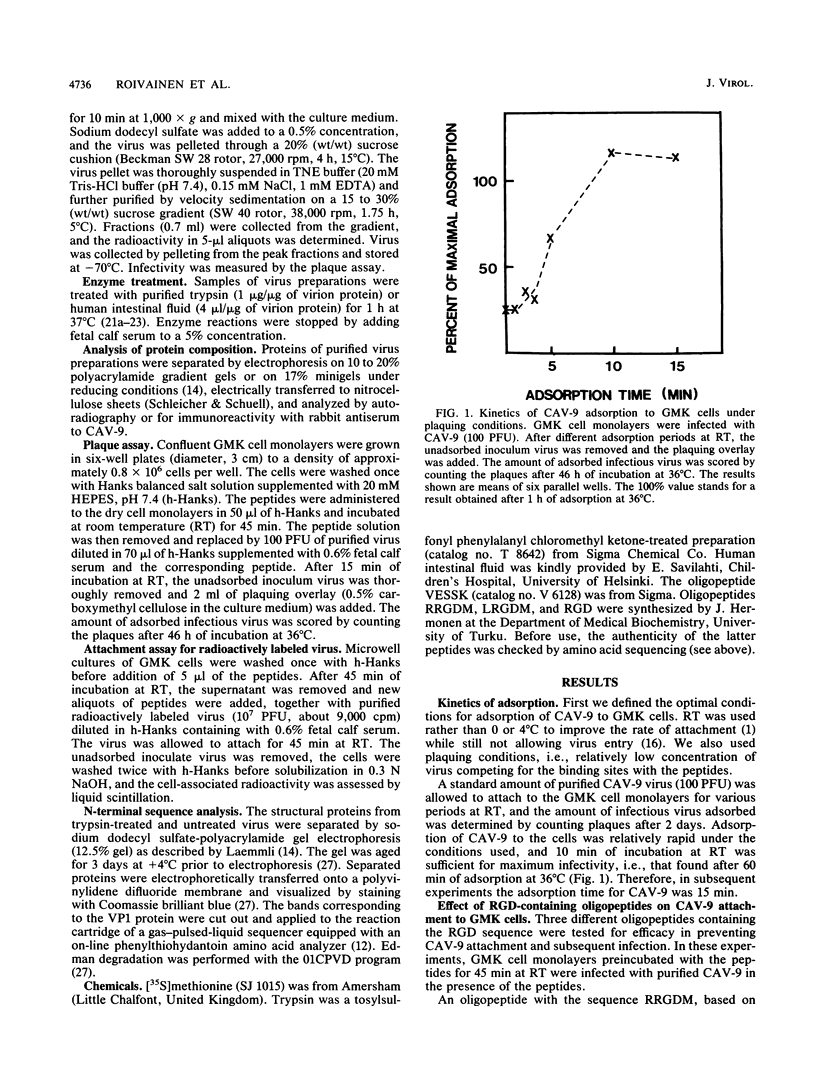
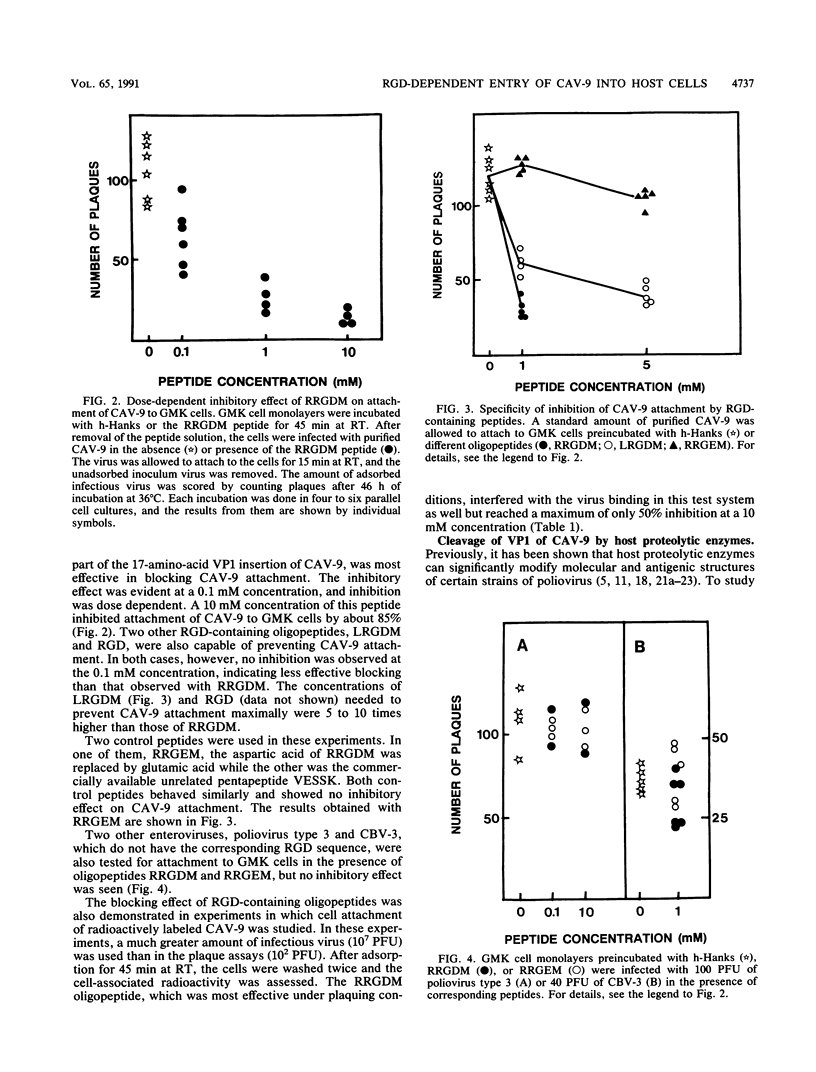
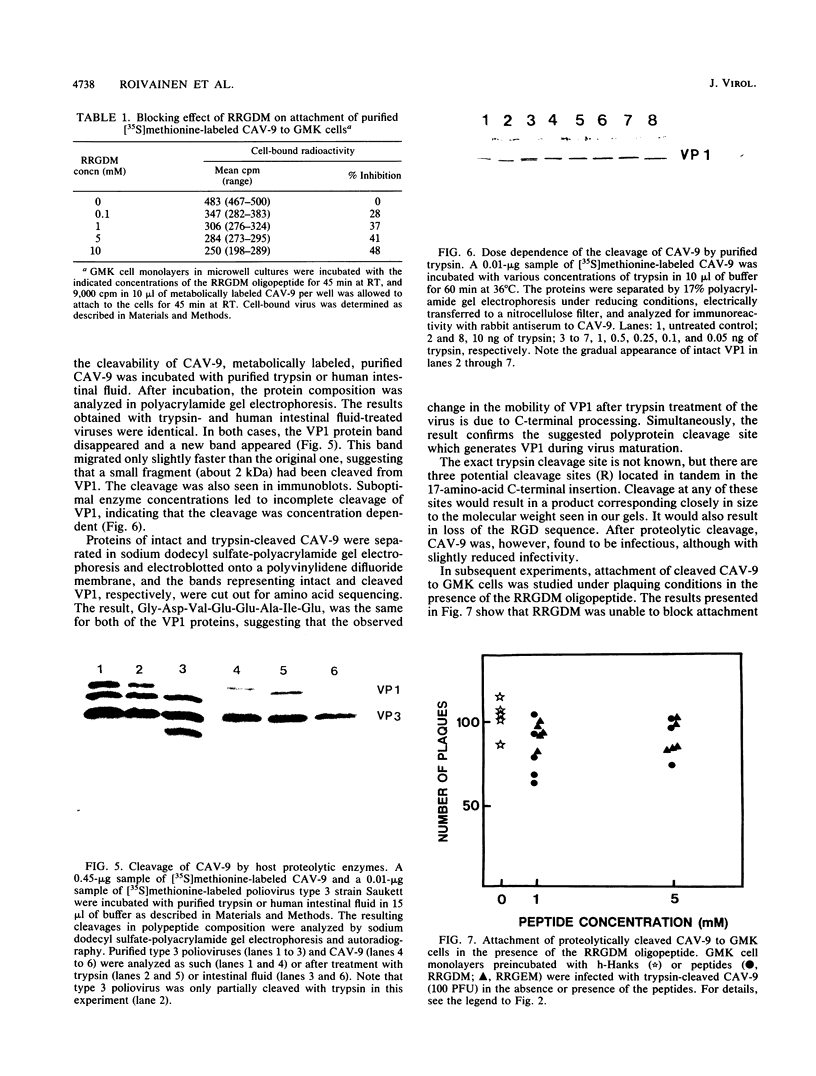
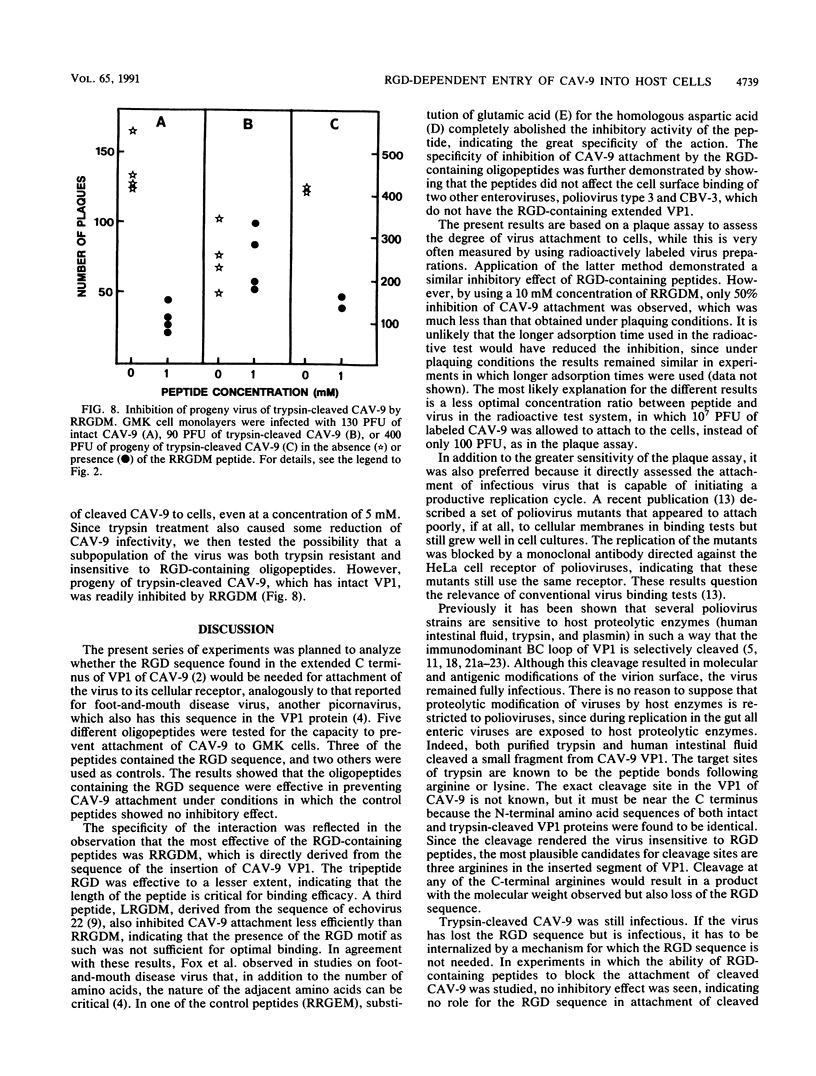
The recently reported nucleotide sequence of coxsackievirus A9 (CAV-9) showed that unlike other enteroviruses, CAV-9 has an insertion of about 17 amino acids at the C-terminal end of VP1 (K. H. Chang, P. Auvinen, T. Hyypiä, and G. Stanway, J. Gen. Virol. 70:3269-3280, 1989). This sequence includes the RGD (arginine-glycine-aspartic acid) motif which is known to be important in certain protein-protein interactions. We studied the inhibitory effect of RGD-containing peptides in the attachment of CAV-9 to African green monkey kidney cells. A peptide corresponding to the RRGDM sequence derived from the inserted segment of CAV-9 was found to block virus attachment effectively, and the inhibition was dose dependent. Substitution of glutamic acid for the homologous aspartic acid completely abolished the inhibitory effect, indicating great specificity of the action. During replication in the gut, all enteroviruses are exposed to host proteolytic enzymes. Exposure of CAV-9 to purified trypsin or human intestinal fluid resulted in selective cleavage of the VP1 capsid protein. Intact and trypsin-cleaved VP1 proteins gave identical N-terminal sequences, indicating that cleavage of VP1 takes place near the C terminus. Attachment of proteolytically cleaved infectious CAV-9 to green monkey kidney cells was not prevented by RGD-containing peptides, indicating that cleaved CAV-9 is able to bypass RGD-dependent entry. The altered receptor specificity of proteolytically cleaved viruses may have important consequences in the pathogenesis of enteric infections.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxt B., Bachrach H. L. Early interactions of foot-and-mouth disease virus with cultured cells. Virology. 1980 Jul 15;104(1):42–55. doi: 10.1016/0042-6822(80)90364-5. [DOI] [PubMed] [Google Scholar]
- Chang K. H., Auvinen P., Hyypiä T., Stanway G. The nucleotide sequence of coxsackievirus A9; implications for receptor binding and enterovirus classification. J Gen Virol. 1989 Dec;70(Pt 12):3269–3280. doi: 10.1099/0022-1317-70-12-3269. [DOI] [PubMed] [Google Scholar]
- Fox G., Parry N. R., Barnett P. V., McGinn B., Rowlands D. J., Brown F. The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartic acid). J Gen Virol. 1989 Mar;70(Pt 3):625–637. doi: 10.1099/0022-1317-70-3-625. [DOI] [PubMed] [Google Scholar]
- Fricks C. E., Icenogle J. P., Hogle J. M. Trypsin sensitivity of the Sabin strain of type 1 poliovirus: cleavage sites in virions and related particles. J Virol. 1985 Jun;54(3):856–859. doi: 10.1128/jvi.54.3.856-859.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Helenius A., Morein B., Fries E., Simons K., Robinson P., Schirrmacher V., Terhorst C., Strominger J. L. Human (HLA-A and HLA-B) and murine (H-2K and H-2D) histocompatibility antigens are cell surface receptors for Semliki Forest virus. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3846–3850. doi: 10.1073/pnas.75.8.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P. J., North C., Minor P. D., Stanway G. The complete nucleotide sequence of coxsackievirus A21. J Gen Virol. 1989 Nov;70(Pt 11):2943–2952. doi: 10.1099/0022-1317-70-11-2943. [DOI] [PubMed] [Google Scholar]
- Icenogle J. P., Minor P. D., Ferguson M., Hogle J. M. Modulation of humoral response to a 12-amino-acid site on the poliovirus virion. J Virol. 1986 Oct;60(1):297–301. doi: 10.1128/jvi.60.1.297-301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Peters D., Racaniello V. R. Poliovirus mutants resistant to neutralization with soluble cell receptors. Science. 1990 Dec 14;250(4987):1596–1599. doi: 10.1126/science.2177226. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mapoles J. E., Krah D. L., Crowell R. L. Purification of a HeLa cell receptor protein for group B coxsackieviruses. J Virol. 1985 Sep;55(3):560–566. doi: 10.1128/jvi.55.3.560-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Minor P. D., Ferguson M., Phillips A., Magrath D. I., Huovilainen A., Hovi T. Conservation in vivo of protease cleavage sites in antigenic sites of poliovirus. J Gen Virol. 1987 Jul;68(Pt 7):1857–1865. doi: 10.1099/0022-1317-68-7-1857. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Dutko F. J., Kennedy S. I., Holland J. J., Lampert P. W. Does the major histocompatibility complex serve as a specific receptor for Semliki Forest virus? J Virol. 1980 Apr;34(1):256–265. doi: 10.1128/jvi.34.1.256-265.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan K. J., Goldberg B., Crowell R. L. Altered receptor specificity of coxsackievirus B3 after growth in rhabdomyosarcoma cells. J Virol. 1984 Mar;49(3):635–640. doi: 10.1128/jvi.49.3.635-640.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roivainen M., Hovi T. Cleavage of VP1 and modification of antigenic site 1 of type 2 polioviruses by intestinal trypsin. J Virol. 1988 Sep;62(9):3536–3539. doi: 10.1128/jvi.62.9.3536-3539.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roivainen M., Hovi T. Intestinal trypsin can significantly modify antigenic properties of polioviruses: implications for the use of inactivated poliovirus vaccine. J Virol. 1987 Dec;61(12):3749–3753. doi: 10.1128/jvi.61.12.3749-3753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roivainen M., Huovilainen A., Hovi T. Antigenic modification of polioviruses by host proteolytic enzymes. Arch Virol. 1990;111(1-2):115–125. doi: 10.1007/BF01310509. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- Svensson U. Role of vesicles during adenovirus 2 internalization into HeLa cells. J Virol. 1985 Aug;55(2):442–449. doi: 10.1128/jvi.55.2.442-449.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]





