Abstract
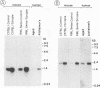
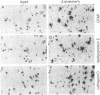
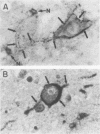
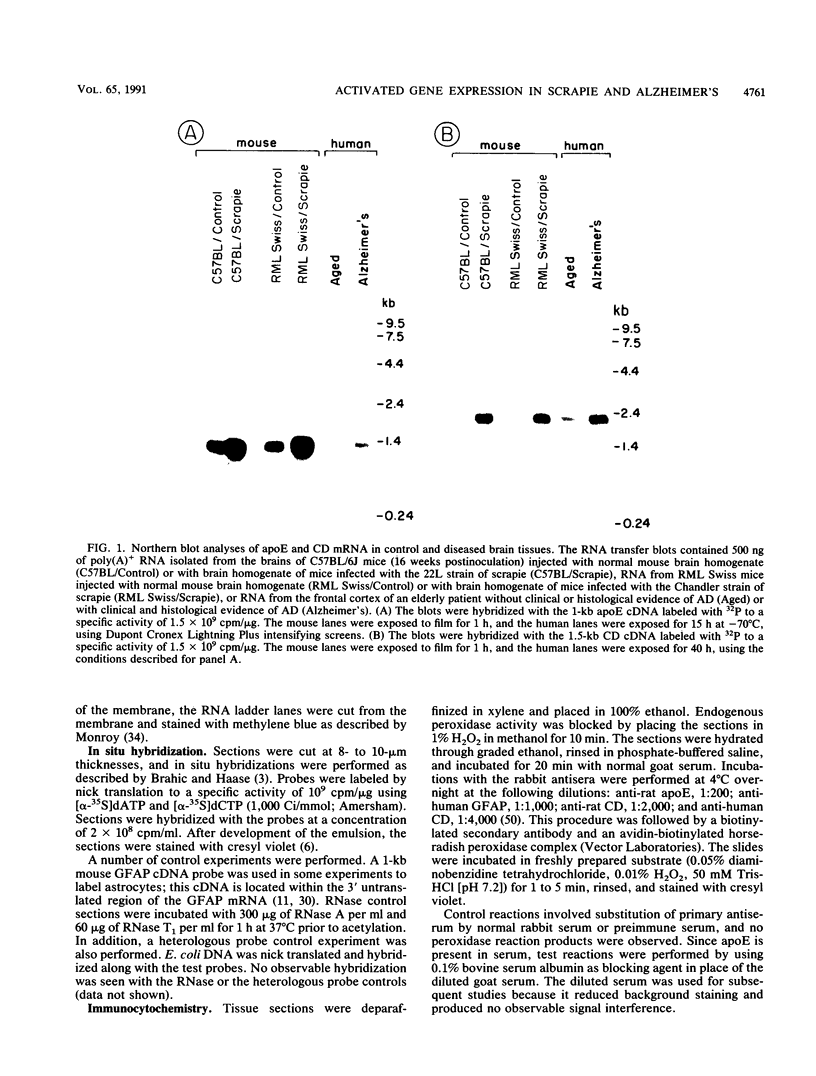
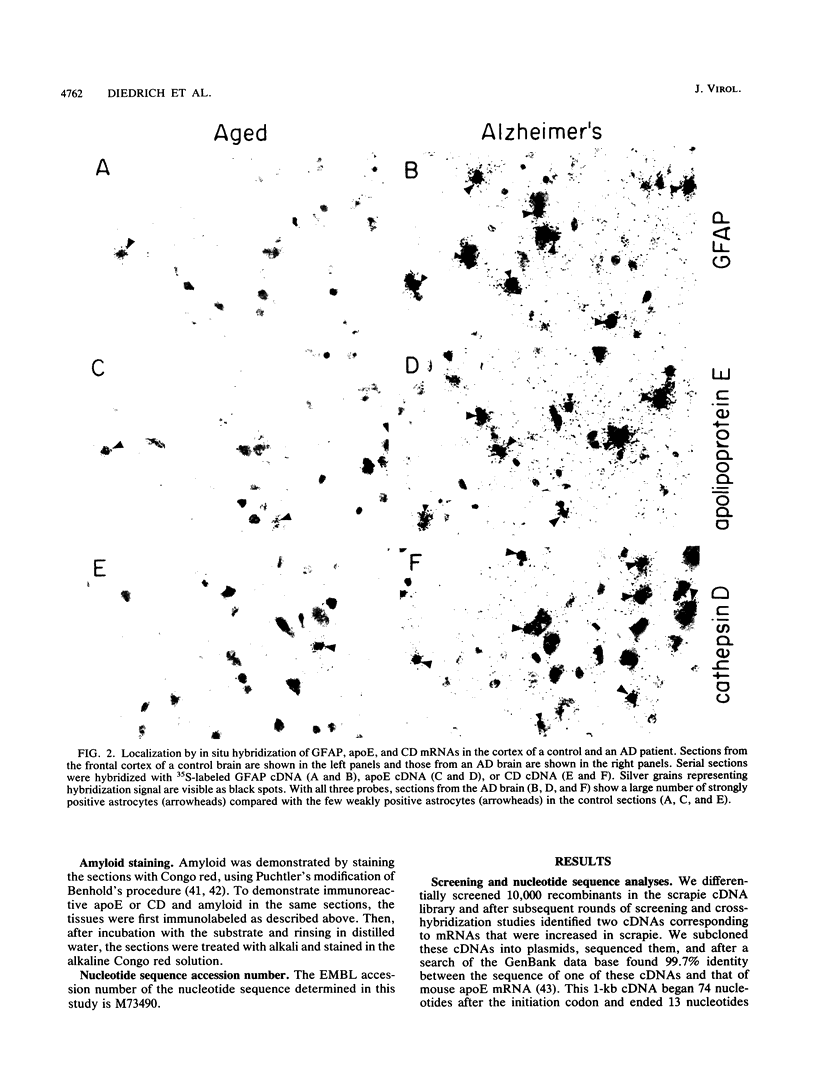
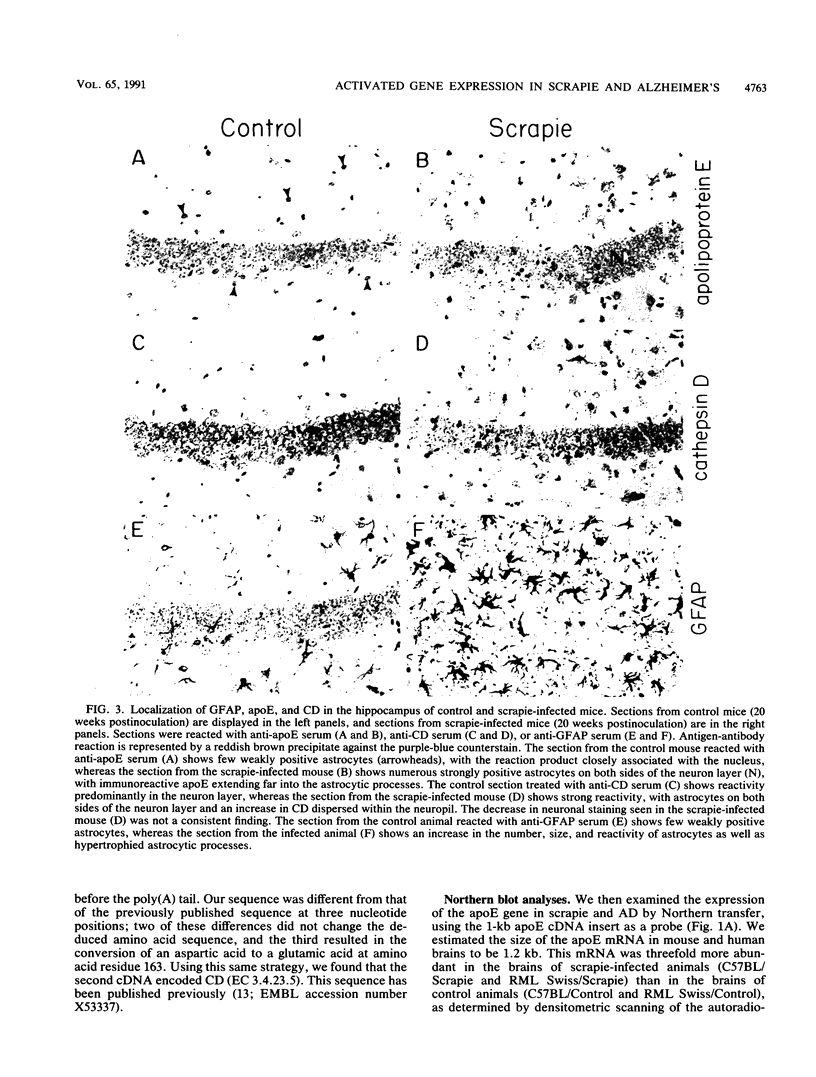
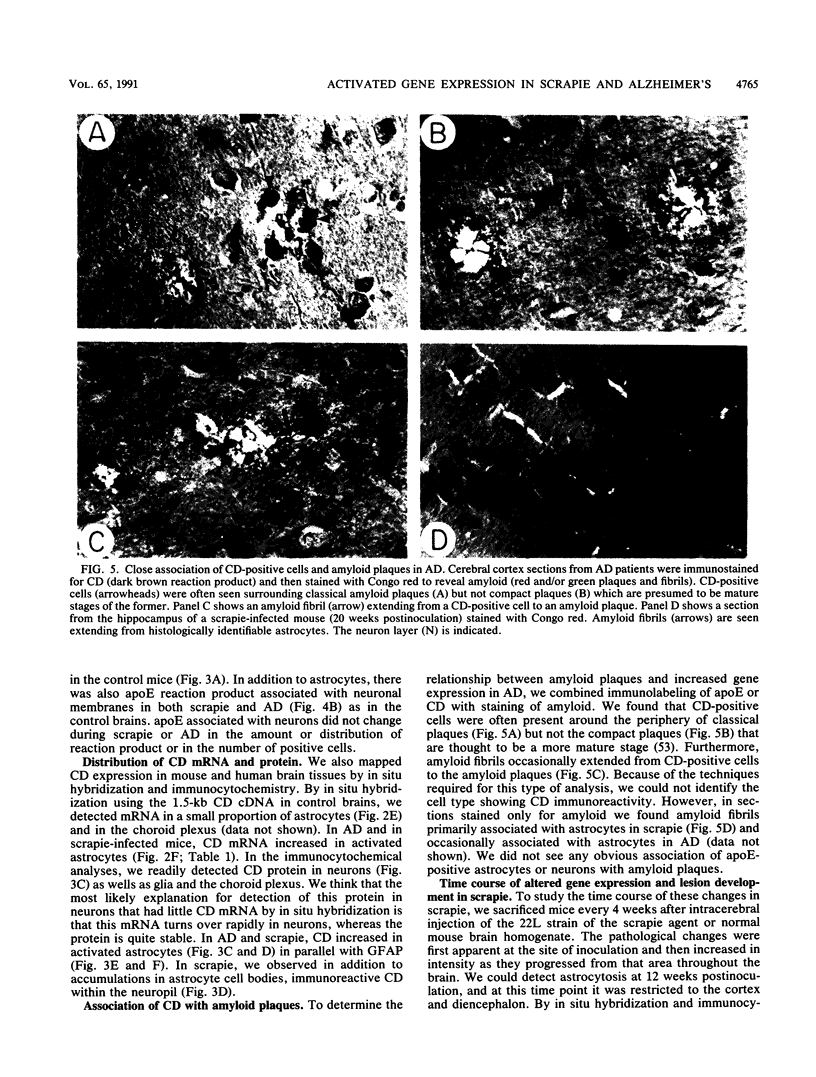
With the rationale that the neuropathological similarities between scrapie and Alzheimer's disease reflect convergent pathological mechanisms involving altered gene expression, we set out to identify molecular events involved in both processes, using scrapie as a model to study the time course of these changes. We differentially screened a cDNA library constructed from scrapie-infected mice to identify mRNAs that increase or decrease during disease and discovered in this way two mRNAs that are increased in scrapie and Alzheimer's disease. These mRNAs were subsequently shown by sequence analysis to encode apolipoprotein E and cathepsin D (EC 3.4.23.5). Using in situ hybridization and immunocytochemistry to define the cellular and anatomic pathology of altered gene expression, we found that in both diseases the increase in apolipoprotein E and cathepsin D mRNAs and proteins occurred in activated astrocytes. In scrapie, the increase in gene expression occurred soon after the amyloid-forming abnormal isoform of the prion protein has been shown to accumulate in astrocytes. In Alzheimer's disease, the increased expression of cathepsin D also occurred in association with beta-amyloid. These studies reveal some of the molecular antecedents of neuropathological changes in scrapie and Alzheimer's disease and accord new prominence to the role of astrocytes in neurodegenerative conditions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendheim P. E., Barry R. A., DeArmond S. J., Stites D. P., Prusiner S. B. Antibodies to a scrapie prion protein. Nature. 1984 Aug 2;310(5976):418–421. doi: 10.1038/310418a0. [DOI] [PubMed] [Google Scholar]
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M. E., McBride P. A., Farquhar C. F. Precise targeting of the pathology of the sialoglycoprotein, PrP, and vacuolar degeneration in mouse scrapie. Neurosci Lett. 1989 Jul 17;102(1):1–6. doi: 10.1016/0304-3940(89)90298-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell A. H., Finkbeiner S. M., Cooper M. S., Smith S. J. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990 Jan 26;247(4941):470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- DeArmond S. J., McKinley M. P., Barry R. A., Braunfeld M. B., McColloch J. R., Prusiner S. B. Identification of prion amyloid filaments in scrapie-infected brain. Cell. 1985 May;41(1):221–235. doi: 10.1016/0092-8674(85)90076-5. [DOI] [PubMed] [Google Scholar]
- DeArmond S. J., Mobley W. C., DeMott D. L., Barry R. A., Beckstead J. H., Prusiner S. B. Changes in the localization of brain prion proteins during scrapie infection. Neurology. 1987 Aug;37(8):1271–1280. doi: 10.1212/wnl.37.8.1271. [DOI] [PubMed] [Google Scholar]
- Diedrich J. F., Bendheim P. E., Kim Y. S., Carp R. I., Haase A. T. Scrapie-associated prion protein accumulates in astrocytes during scrapie infection. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):375–379. doi: 10.1073/pnas.88.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich J. F., Staskus K. A., Retzel E. F., Haase A. T. Nucleotide sequence of a cDNA encoding mouse cathepsin D. Nucleic Acids Res. 1990 Dec 11;18(23):7184–7184. doi: 10.1093/nar/18.23.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich J., Wietgrefe S., Zupancic M., Staskus K., Retzel E., Haase A. T., Race R. The molecular pathogenesis of astrogliosis in scrapie and Alzheimer's disease. Microb Pathog. 1987 Jun;2(6):435–442. doi: 10.1016/0882-4010(87)90050-7. [DOI] [PubMed] [Google Scholar]
- Duguid J. R., Bohmont C. W., Liu N. G., Tourtellotte W. W. Changes in brain gene expression shared by scrapie and Alzheimer disease. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7260–7264. doi: 10.1073/pnas.86.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. R., Rohwer R. G., Seed B. Isolation of cDNAs of scrapie-modulated RNAs by subtractive hybridization of a cDNA library. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5738–5742. doi: 10.1073/pnas.85.15.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Lewis E., Wietgrefe S., Zupancic M., Diedrich J., Minnigan H., Ball M. J. Speculations on the role of transmissible agents in the pathogenesis of Alzheimer's disease. Can J Neurol Sci. 1986 Nov;13(4 Suppl):449–451. doi: 10.1017/s0317167100037100. [DOI] [PubMed] [Google Scholar]
- Hadlow W. J., Prusiner S. B., Kennedy R. C., Race R. E. Brain tissue from persons dying of Creutzfeldt-Jakob disease causes scrapie-like encephalopathy in goats. Ann Neurol. 1980 Dec;8(6):628–632. doi: 10.1002/ana.410080615. [DOI] [PubMed] [Google Scholar]
- Hope J., Multhaup G., Reekie L. J., Kimberlin R. H., Beyreuther K. Molecular pathology of scrapie-associated fibril protein (PrP) in mouse brain affected by the ME7 strain of scrapie. Eur J Biochem. 1988 Mar 1;172(2):271–277. doi: 10.1111/j.1432-1033.1988.tb13883.x. [DOI] [PubMed] [Google Scholar]
- Ignatius M. J., Gebicke-Haerter P. J., Pitas R. E., Shooter E. M. Apolipoprotein E in nerve injury and repair. Prog Brain Res. 1987;71:177–184. doi: 10.1016/s0079-6123(08)61822-1. [DOI] [PubMed] [Google Scholar]
- Ignatius M. J., Gebicke-Härter P. J., Skene J. H., Schilling J. W., Weisgraber K. H., Mahley R. W., Shooter E. M. Expression of apolipoprotein E during nerve degeneration and regeneration. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1125–1129. doi: 10.1073/pnas.83.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kascsak R. J., Rubenstein R., Merz P. A., Carp R. I., Wisniewski H. M., Diringer H. Biochemical differences among scrapie-associated fibrils support the biological diversity of scrapie agents. J Gen Virol. 1985 Aug;66(Pt 8):1715–1722. doi: 10.1099/0022-1317-66-8-1715. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Carp R. I., Callahan S. M., Wisniewski H. M. Incubation periods and survival times for mice injected stereotaxically with three scrapie strains in different brain regions. J Gen Virol. 1987 Mar;68(Pt 3):695–702. doi: 10.1099/0022-1317-68-3-695. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Balcarek J. M., Krek V., Shelanski M., Cowan N. J. Sequence of a cDNA clone encoding mouse glial fibrillary acidic protein: structural conservation of intermediate filaments. Proc Natl Acad Sci U S A. 1984 May;81(9):2743–2746. doi: 10.1073/pnas.81.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberski P. P., Yanagihara R., Gibbs C. J., Jr, Gajdusek D. C. Neuroaxonal dystrophy: an ultrastructural link between subacute spongiform virus encephalopathies and Alzheimer's disease. Prog Clin Biol Res. 1989;317:549–557. [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. W., Gebicke-Härter P. J., Hangen D. H., Shooter E. M. A specific 37,000-dalton protein that accumulates in regenerating but not in nonregenerating mammalian nerves. Science. 1985 Apr 26;228(4698):499–501. doi: 10.1126/science.3983637. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panter S. S., McSwigan J. D., Sheppard J. R., Emory C. R., Frey W. H., 2nd Glial fibrillary acidic protein and Alzheimer's disease. Neurochem Res. 1985 Dec;10(12):1567–1576. doi: 10.1007/BF00988599. [DOI] [PubMed] [Google Scholar]
- Perry G., Siedlak S., Mulvihill P., Kancherla M., Mijares M., Kawai M., Gambetti P., Sharma S., Maggiora L., Cornette J. Immunolocalization of the amyloid precursor protein within the senile plaque. Prog Clin Biol Res. 1989;317:1021–1025. [PubMed] [Google Scholar]
- Piccardo P., Safar J., Ceroni M., Gajdusek D. C., Gibbs C. J., Jr Immunohistochemical localization of prion protein in spongiform encephalopathies and normal brain tissue. Neurology. 1990 Mar;40(3 Pt 1):518–522. doi: 10.1212/wnl.40.3_part_1.518. [DOI] [PubMed] [Google Scholar]
- Puchtler H., Sweat F. Congo red as a stain for fluorescence microscopy of amyloid. J Histochem Cytochem. 1965 Nov-Dec;13(8):693–694. doi: 10.1177/13.8.693. [DOI] [PubMed] [Google Scholar]
- Rajavashisth T. B., Kaptein J. S., Reue K. L., Lusis A. J. Evolution of apolipoprotein E: mouse sequence and evidence for an 11-nucleotide ancestral unit. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8085–8089. doi: 10.1073/pnas.82.23.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein R., Kascsak R. J., Merz P. A., Papini M. C., Carp R. I., Robakis N. K., Wisniewski H. M. Detection of scrapie-associated fibril (SAF) proteins using anti-SAF antibody in non-purified tissue preparations. J Gen Virol. 1986 Apr;67(Pt 4):671–681. doi: 10.1099/0022-1317-67-4-671. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R., Card J. P., Nelson R. B., Davis L. G. Expression of beta-amyloid precursor protein in reactive astrocytes following neuronal damage. Neuron. 1989 Sep;3(3):275–285. doi: 10.1016/0896-6273(89)90252-3. [DOI] [PubMed] [Google Scholar]
- Somerville R. A., Ritchie L. A., Gibson P. H. Structural and biochemical evidence that scrapie-associated fibrils assemble in vivo. J Gen Virol. 1989 Jan;70(Pt 1):25–35. doi: 10.1099/0022-1317-70-1-25. [DOI] [PubMed] [Google Scholar]
- Stoll G., Müller H. W. Macrophages in the peripheral nervous system and astroglia in the central nervous system of rat commonly express apolipoprotein E during development but differ in their response to injury. Neurosci Lett. 1986 Dec 23;72(3):233–238. doi: 10.1016/0304-3940(86)90519-7. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietgrefe S., Zupancic M., Haase A., Chesebro B., Race R., Frey W., 2nd, Rustan T., Friedman R. L. Cloning of a gene whose expression is increased in scrapie and in senile plaques in human brain. Science. 1985 Dec 6;230(4730):1177–1179. doi: 10.1126/science.3840915. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Burrola P. G., Buchmeier M. J., Wooddell M. K., Barry R. A., Prusiner S. B., Lampert P. W. Immuno-gold localization of prion filaments in scrapie-infected hamster brains. Lab Invest. 1987 Dec;57(6):646–656. [PubMed] [Google Scholar]
- Yankner B. A., Duffy L. K., Kirschner D. A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990 Oct 12;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]