Abstract
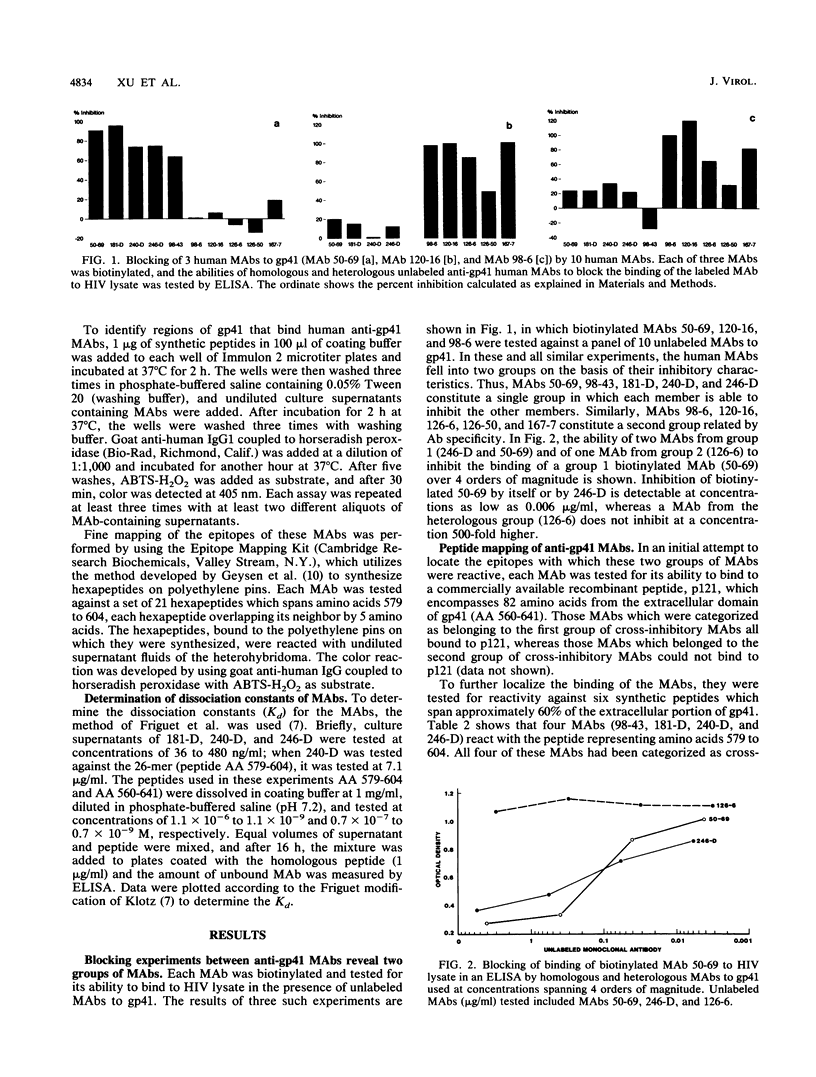
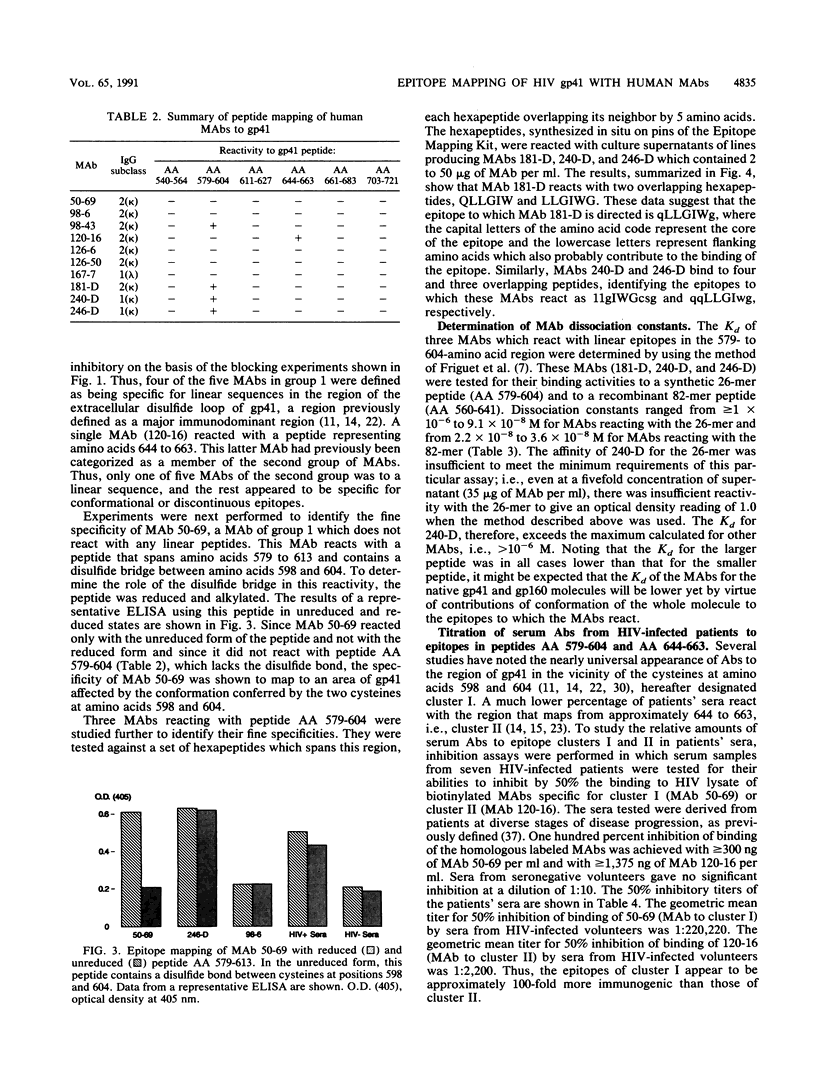
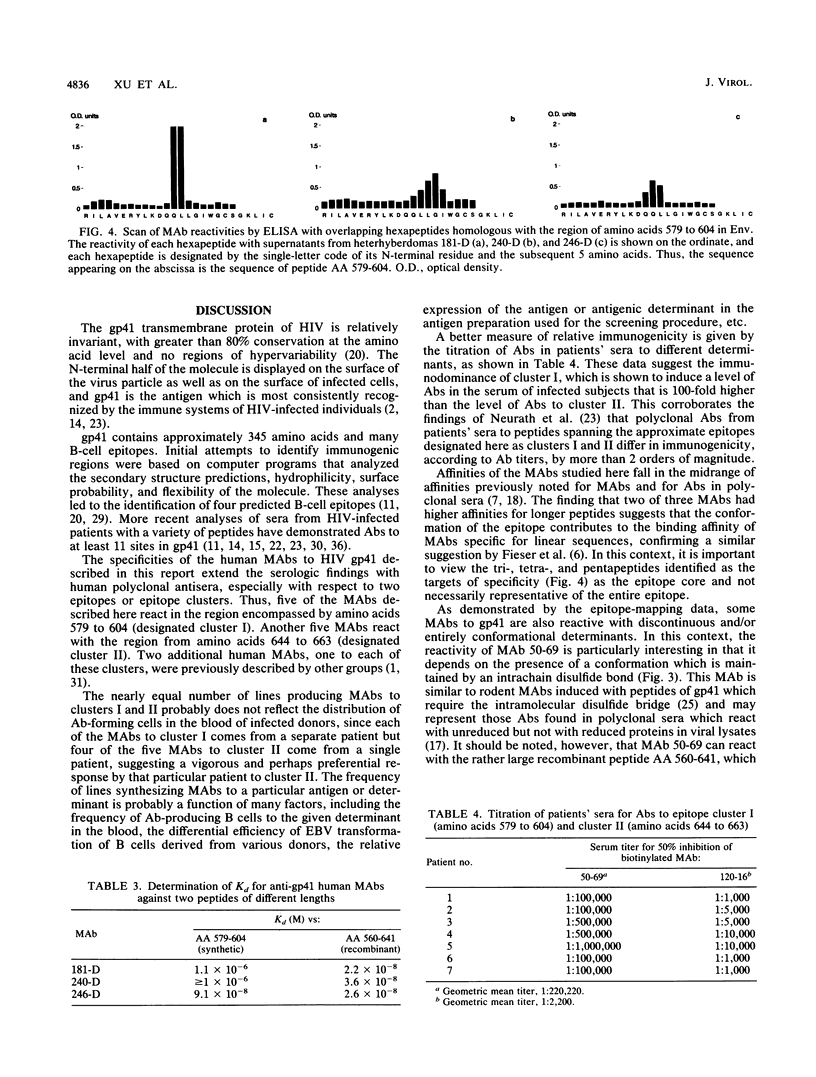
Immunogenic regions of the gp41 transmembrane protein of human immunodeficiency virus type 1 (HIV-1) were previously mapped by examining polyclonal sera from HIV-infected patients and rodent polyclonal and monoclonal antibodies (MAbs) to peptides of gp41. To define the epitopes within these regions to which infected humans respond during the course of infection, the specificity of human MAbs to these regions had to be studied. Using 10 human MAbs identified initially by their reactivity to whole gp41 in HIV-1 lysates, the epitopes within the immunodominant region of gp41 and within a second immunogenic region of gp41 have been mapped. Thus, five MAbs (from five different patients) to the immunodominant domain of gp41 in the vicinity of the cysteines at positions 598 and 604 (hereinafter designated cluster I) reacted with a stretch of 11 amino acids from positions 590 to 600. Four of these five MAbs were reactive with linear epitopes, while one MAb required the conformation conferred by the disulfide bridge between the aforementioned cysteines. Three MAbs to cluster I revealed dissociation constants ranging from 10(-6) to 10(-8) M, depending on the MAb tested and the size of the synthetic or recombinant peptide used in the assay. Five additional MAbs reacted with a second immunogenic region between positions 644 and 663 (designated cluster II). Four of these five MAbs were specific for conformational determinants. Titration of sera from HIV-infected patients showed that there was about 100-fold more antibody to cluster I than to cluster II in patients' sera, confirming the immunodominance of cluster I.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banapour B., Rosenthal K., Rabin L., Sharma V., Young L., Fernandez J., Engleman E., McGrath M., Reyes G., Lifson J. Characterization and epitope mapping of a human monoclonal antibody reactive with the envelope glycoprotein of human immunodeficiency virus. J Immunol. 1987 Dec 15;139(12):4027–4033. [PubMed] [Google Scholar]
- Barin F., McLane M. F., Allan J. S., Lee T. H., Groopman J. E., Essex M. Virus envelope protein of HTLV-III represents major target antigen for antibodies in AIDS patients. Science. 1985 May 31;228(4703):1094–1096. doi: 10.1126/science.2986291. [DOI] [PubMed] [Google Scholar]
- Bost K. L., Pascual D. W. Antibodies against a peptide sequence within the HIV envelope protein crossreacts with human interleukin-2. Immunol Invest. 1988 Aug-Oct;17(6-7):577–586. doi: 10.3109/08820138809030591. [DOI] [PubMed] [Google Scholar]
- Chanh T. C., Dreesman G. R., Kanda P., Linette G. P., Sparrow J. T., Ho D. D., Kennedy R. C. Induction of anti-HIV neutralizing antibodies by synthetic peptides. EMBO J. 1986 Nov;5(11):3065–3071. doi: 10.1002/j.1460-2075.1986.tb04607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., McKeating J., Meredith J. M., Burke K. L., Katrak K., John A., Ferguson M., Minor P. D., Weiss R. A., Almond J. W. An engineered poliovirus chimaera elicits broadly reactive HIV-1 neutralizing antibodies. Nature. 1989 Jun 1;339(6223):385-8, 340. doi: 10.1038/339385a0. [DOI] [PubMed] [Google Scholar]
- Fieser T. M., Tainer J. A., Geysen H. M., Houghten R. A., Lerner R. A. Influence of protein flexibility and peptide conformation on reactivity of monoclonal anti-peptide antibodies with a protein alpha-helix. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8568–8572. doi: 10.1073/pnas.84.23.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friguet B., Chaffotte A. F., Djavadi-Ohaniance L., Goldberg M. E. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods. 1985 Mar 18;77(2):305–319. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- Gallaher W. R., Ball J. M., Garry R. F., Griffin M. C., Montelaro R. C. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res Hum Retroviruses. 1989 Aug;5(4):431–440. doi: 10.1089/aid.1989.5.431. [DOI] [PubMed] [Google Scholar]
- Gallaher W. R. Detection of a fusion peptide sequence in the transmembrane protein of human immunodeficiency virus. Cell. 1987 Jul 31;50(3):327–328. doi: 10.1016/0092-8674(87)90485-5. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnann J. W., Jr, Schwimmbeck P. L., Nelson J. A., Truax A. B., Oldstone M. B. Diagnosis of AIDS by using a 12-amino acid peptide representing an immunodominant epitope of the human immunodeficiency virus. J Infect Dis. 1987 Aug;156(2):261–267. doi: 10.1093/infdis/156.2.261. [DOI] [PubMed] [Google Scholar]
- Golding H., Robey F. A., Gates F. T., 3rd, Linder W., Beining P. R., Hoffman T., Golding B. Identification of homologous regions in human immunodeficiency virus I gp41 and human MHC class II beta 1 domain. I. Monoclonal antibodies against the gp41-derived peptide and patients' sera react with native HLA class II antigens, suggesting a role for autoimmunity in the pathogenesis of acquired immune deficiency syndrome. J Exp Med. 1988 Mar 1;167(3):914–923. doi: 10.1084/jem.167.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny M. K., Gianakakos V., Sharpe S., Zolla-Pazner S. Generation of human monoclonal antibodies to human immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1624–1628. doi: 10.1073/pnas.86.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit J., Meloen R. H., Brasseur R. Map of sequential B cell epitopes of the HIV-1 transmembrane protein using human antibodies as probe. Intervirology. 1990;31(6):327–338. doi: 10.1159/000150169. [DOI] [PubMed] [Google Scholar]
- Klasse P. J., Pipkorn R., Blomberg J. Presence of antibodies to a putatively immunosuppressive part of human immunodeficiency virus (HIV) envelope glycoprotein gp41 is strongly associated with health among HIV-positive subjects. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5225–5229. doi: 10.1073/pnas.85.14.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski M., Potz J., Basiripour L., Dorfman T., Goh W. C., Terwilliger E., Dayton A., Rosen C., Haseltine W., Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987 Sep 11;237(4820):1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- Larsson A., Ghosh R., Hammarström S. Determination of intrinsic affinity constants of monoclonal antibodies against carcinoembryonic antigen. Mol Immunol. 1987 Jun;24(6):569–576. doi: 10.1016/0161-5890(87)90037-x. [DOI] [PubMed] [Google Scholar]
- Liu Y. S., Green A. A monoclonal-antibody enzyme immunoassay for detection of hepatitis B surface antigen with use of a biotin-avidin system. Clin Chem. 1985 Feb;31(2):202–205. [PubMed] [Google Scholar]
- Modrow S., Hahn B. H., Shaw G. M., Gallo R. C., Wong-Staal F., Wolf H. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J Virol. 1987 Feb;61(2):570–578. doi: 10.1128/jvi.61.2.570-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath A. R., Strick N., Taylor P., Rubinstein P., Stevens C. E. Search for epitope-specific antibody responses to the human immunodeficiency virus (HIV-1) envelope glycoproteins signifying resistance to disease development. AIDS Res Hum Retroviruses. 1990 Oct;6(10):1183–1192. doi: 10.1089/aid.1990.6.1183. [DOI] [PubMed] [Google Scholar]
- Norrby E., Parks D. E., Utter G., Houghten R. A., Lerner R. A. Immunochemistry of the dominating antigenic region Ala582 to Cys604 in the transmembranous protein of simian and human immunodeficiency virus. J Immunol. 1989 Dec 1;143(11):3602–3608. [PubMed] [Google Scholar]
- Närvänen A., Korkolainen M., Kontio S., Suni J., Turtianen S., Partanen P., Soos J., Vaheri A., Huhtala M. L. Highly immunoreactive antigenic site in a hydrophobic domain of HIV-1 gp41 which remains undetectable with conventional immunochemical methods. AIDS. 1988 Apr;2(2):119–123. doi: 10.1097/00002030-198804000-00008. [DOI] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Wilson C., Naugle C., Gallo R. C., Robert-Guroff M. Generation of a neutralization-resistant variant of HIV-1 is due to selection for a point mutation in the envelope gene. Cell. 1988 Jul 1;54(1):57–63. doi: 10.1016/0092-8674(88)90179-1. [DOI] [PubMed] [Google Scholar]
- Robinson W. E., Jr, Gorny M. K., Xu J. Y., Mitchell W. M., Zolla-Pazner S. Two immunodominant domains of gp41 bind antibodies which enhance human immunodeficiency virus type 1 infection in vitro. J Virol. 1991 Aug;65(8):4169–4176. doi: 10.1128/jvi.65.8.4169-4176.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. E., Jr, Kawamura T., Gorny M. K., Lake D., Xu J. Y., Matsumoto Y., Sugano T., Masuho Y., Mitchell W. M., Hersh E. Human monoclonal antibodies to the human immunodeficiency virus type 1 (HIV-1) transmembrane glycoprotein gp41 enhance HIV-1 infection in vitro. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3185–3189. doi: 10.1073/pnas.87.8.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B., Fishleigh R. V., Morrison C. A. Prediction of HIV vaccine. 1987 Jan 29-Feb 4Nature. 325(6103):395–395. doi: 10.1038/325395a0. [DOI] [PubMed] [Google Scholar]
- Schrier R. D., Gnann J. W., Jr, Langlois A. J., Shriver K., Nelson J. A., Oldstone M. B. B- and T-lymphocyte responses to an immunodominant epitope of human immunodeficiency virus. J Virol. 1988 Aug;62(8):2531–2536. doi: 10.1128/jvi.62.8.2531-2536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeuwsen V. J., Siebelink K. H., Crush-Stanton S., Swerdlow B., Schalken J. J., Goudsmit J., van de Akker R., Stukart M. J., Uytdehaag F. G., Osterhaus A. D. Production and characterization of a human monoclonal antibody, reactive with a conserved epitope on gp41 of human immunodeficiency virus type I. AIDS Res Hum Retroviruses. 1990 Mar;6(3):381–392. doi: 10.1089/aid.1990.6.381. [DOI] [PubMed] [Google Scholar]
- Teng N. N., Lam K. S., Calvo Riera F., Kaplan H. S. Construction and testing of mouse--human heteromyelomas for human monoclonal antibody production. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7308–7312. doi: 10.1073/pnas.80.23.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till M. A., Ghetie V., May R. D., Auerbach P. C., Zolla-Pazner S., Gorny M. K., Gregory T., Uhr J. W., Vitetta E. S. Immunoconjugates containing ricin A chain and either human anti-gp41 or CD4 kill H9 cells infected with different isolates of HIV, but do not inhibit normal T or B cell function. J Acquir Immune Defic Syndr. 1990;3(6):609–614. [PubMed] [Google Scholar]
- Till M. A., Zolla-Pazner S., Gorny M. K., Patton J. S., Uhr J. W., Vitetta E. S. Human immunodeficiency virus-infected T cells and monocytes are killed by monoclonal human anti-gp41 antibodies coupled to ricin A chain. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1987–1991. doi: 10.1073/pnas.86.6.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. S., Stanley S. D., Zolla-Pazner S., Gorny M. K., Shadduck P. P., Langlois A. J., Matthews T. J., Bolognesi D. P., Palker T. J., Weinhold K. J. Identification of sites within gp41 that serve as targets for antibody-dependent cellular cytotoxicity by using human monoclonal antibodies. J Immunol. 1990 Nov 15;145(10):3276–3282. [PubMed] [Google Scholar]
- Zolla-Pazner S., Des Jarlais D. C., Friedman S. R., Spira T. J., Marmor M., Holzman R., Mildvan D., Yancovitz S., Mathur-Wagh U., Garber J. Nonrandom development of immunologic abnormalities after infection with human immunodeficiency virus: implications for immunologic classification of the disease. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5404–5408. doi: 10.1073/pnas.84.15.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]



