Abstract
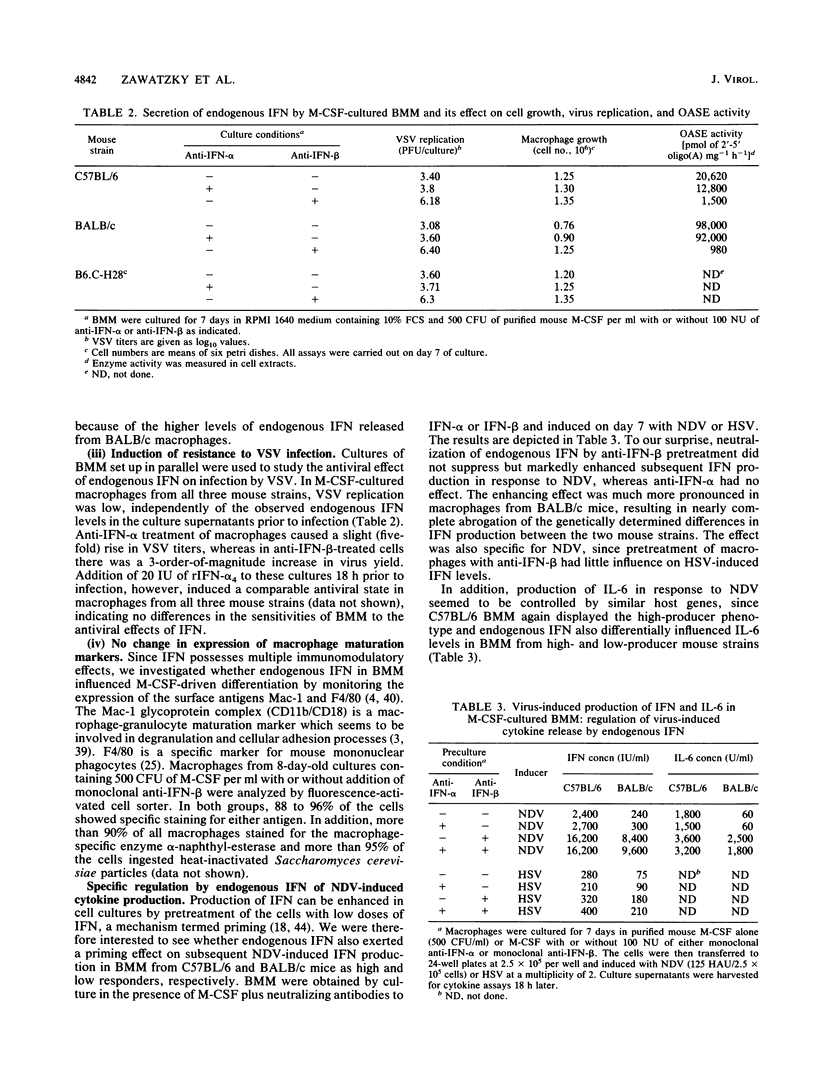
In macrophages from inbred mice, the magnitude of the interferon (IFN) response to Newcastle disease virus (NDV) infection is under genetic control of the If-1 locus, which carries the allele for either high (h) or low (l) IFN production. Here, we report that the activity of genes within the If-1 locus is influenced by macrophage-derived endogenous IFN. In addition to various other biological effects, we observed that endogenous IFN specifically downregulated NDV-induced IFN and interleukin 6 production. Preculture of bone marrow-derived macrophages (BMM) from BALB/c (If-1l) mice in macrophage colony-stimulating factor plus anti-IFN-beta provoked a 30- to 50-fold increase in NDV-induced cytokine production compared with induced control cultures in macrophage colony-stimulating factor alone, whereas only a 4- to 6-fold increase was observed in anti-IFN-beta-treated BMM from C57BL/6 (If-1h) mice. This resulted in nearly complete abrogation of the genetically determined difference in the response to NDV. The increase was specific for NDV and was marked by strong additional activation of IFN-alpha genes. Studies using BMM from B6.C-H28c If-1l congenic mice gave results identical to those obtained with BALB/c BMM. Addition of 20 IU of recombinant IFN-alpha 4 to anti IFN-beta-treated macrophages from B6.C-H28c mice 20 h prior to NDV infection strongly downregulated the IFN-alpha, IFN-beta, and interleukin 6 responses. The genetic difference between macrophages from If-1h and If-1l mice was thus reestablished, since the same treatment caused only weak reduction of NDV-induced cytokine gene expression in BMM from C57BL/6 mice. These data suggest that the If-1h and If-1l alleles harbor IFN-inducible genes that, following activation, specifically suppress subsequent cytokine gene expression in response to NDV.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Aebi M., Fäh J., Hurt N., Samuel C. E., Thomis D., Bazzigher L., Pavlovic J., Haller O., Staeheli P. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol Cell Biol. 1989 Nov;9(11):5062–5072. doi: 10.1128/mcb.9.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., Miller L. J., Schmalstieg F. C., Rothlein R., Springer T. A. Contributions of the Mac-1 glycoprotein family to adherence-dependent granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. J Immunol. 1986 Jul 1;137(1):15–27. [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Bacon T. H., de Vere-Tyndall A., Tyrrell D. A., Denman A. M., Ansell B. M. Interferon system in patients with systemic juvenile chronic arthritis: in vivo and in vitro studies. Clin Exp Immunol. 1983 Oct;54(1):23–30. [PMC free article] [PubMed] [Google Scholar]
- Belardelli F., Vignaux F., Proietti E., Gresser I. Injection of mice with antibody to interferon renders peritoneal macrophages permissive for vesicular stomatitis virus and encephalomyocarditis virus. Proc Natl Acad Sci U S A. 1984 Jan;81(2):602–606. doi: 10.1073/pnas.81.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursnell M. E., Green P. F., Campbell J. I., Deuter A., Peters R. W., Tomley F. M., Samson A. C., Chambers P., Emmerson P. T., Binns M. M. Insertion of the fusion gene from Newcastle disease virus into a non-essential region in the terminal repeats of fowlpox virus and demonstration of protective immunity induced by the recombinant. J Gen Virol. 1990 Mar;71(Pt 3):621–628. doi: 10.1099/0022-1317-71-3-621. [DOI] [PubMed] [Google Scholar]
- Chambers P., Millar N. S., Bingham R. W., Emmerson P. T. Molecular cloning of complementary DNA to Newcastle disease virus, and nucleotide sequence analysis of the junction between the genes encoding the haemagglutinin-neuraminidase and the large protein. J Gen Virol. 1986 Mar;67(Pt 3):475–486. doi: 10.1099/0022-1317-67-3-475. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dandoy F., de Maeyer E., de Maeyer-Guignard J. Antiproliferative action of interferon on murine bone-marrow derived macrophages is influence by the genotype of the marrow-donor. J Interferon Res. 1981 Feb;1(2):263–270. doi: 10.1089/jir.1981.1.263. [DOI] [PubMed] [Google Scholar]
- Falcoff R., Falcoff E. Induction de la synthèse d'interféron par des RNA bicaténaires. II. Etude de la forme intermédiaire de réplication du virus Mengo. Biochim Biophys Acta. 1970 Jan 21;199(1):147–158. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fujita T., Kohno S. Studies on interferon priming: cellular response to viral and nonviral inducers and requirement of protein synthesis. Virology. 1981 Jul 15;112(1):62–69. doi: 10.1016/0042-6822(81)90612-7. [DOI] [PubMed] [Google Scholar]
- Goetschy J. F., Zeller H., Content J., Horisberger M. A. Regulation of the interferon-inducible IFI-78K gene, the human equivalent of the murine Mx gene, by interferons, double-stranded RNA, certain cytokines, and viruses. J Virol. 1989 Jun;63(6):2616–2622. doi: 10.1128/jvi.63.6.2616-2622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Vignaux F., Belardelli F., Tovey M. G., Maunoury M. T. Injection of mice with antibody to mouse interferon alpha/beta decreases the level of 2'-5' oligoadenylate synthetase in peritoneal macrophages. J Virol. 1985 Jan;53(1):221–227. doi: 10.1128/jvi.53.1.221-227.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y., Sokawa Y., Watanabe Y., Kawade Y., Ohno S., Takaoka C., Taniguchi T. Structure and expression of a cloned cDNA for mouse interferon-beta. J Biol Chem. 1983 Aug 10;258(15):9522–9529. [PubMed] [Google Scholar]
- Hirsch S., Austyn J. M., Gordon S. Expression of the macrophage-specific antigen F4/80 during differentiation of mouse bone marrow cells in culture. J Exp Med. 1981 Sep 1;154(3):713–725. doi: 10.1084/jem.154.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoss-Homfeld A., Zwarthoff E. C., Zawatzky R. Cell type specific expression and regulation of murine interferon alpha and beta genes. Virology. 1989 Dec;173(2):539–550. doi: 10.1016/0042-6822(89)90566-7. [DOI] [PubMed] [Google Scholar]
- Hoss A., Zwarthoff E. C., Zawatzky R. Differential expression of interferon alpha and beta induced with Newcastle disease virus in mouse macrophage cultures. J Gen Virol. 1989 Mar;70(Pt 3):575–589. doi: 10.1099/0022-1317-70-3-575. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Gordon S. Mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Identification of resident macrophages in renal medullary and cortical interstitium and the juxtaglomerular complex. J Exp Med. 1983 May 1;157(5):1704–1709. doi: 10.1084/jem.157.5.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M., Cayley P. J., Silverman R. H., Wreschner D. H., Gilbert C. S., Brown R. E., Kerr I. M. Radioimmune, radiobinding and HPLC analysis of 2-5A and related oligonucleotides from intact cells. Nature. 1980 Nov 13;288(5787):189–192. doi: 10.1038/288189a0. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Shaw M., Broni B., Shapiro G., Haller O. Inhibition of influenza viral mRNA synthesis in cells expressing the interferon-induced Mx gene product. J Virol. 1985 Oct;56(1):201–206. doi: 10.1128/jvi.56.1.201-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse A., Kirchner H., Zawatzky R., Domke-Opitz I. In vitro development of bone-marrow-derived macrophages. Influence of mouse genotype on response to colony-stimulating factors and autocrine interferon induction. Scand J Immunol. 1989 Dec;30(6):731–740. doi: 10.1111/j.1365-3083.1989.tb02483.x. [DOI] [PubMed] [Google Scholar]
- Le J. M., Vilcek J. Interleukin 6: a multifunctional cytokine regulating immune reactions and the acute phase protein response. Lab Invest. 1989 Dec;61(6):588–602. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Fuller F. J. Interferon induction by viruses. II. Sindbis virus: interferon induction requires one-quarter of the genome--genes G and A. J Gen Virol. 1979 Jul;44(1):169–177. doi: 10.1099/0022-1317-44-1-169. [DOI] [PubMed] [Google Scholar]
- Moore R. N., Larsen H. S., Horohov D. W., Rouse B. T. Endogenous regulation of macrophage proliferative expansion by colony-stimulating factor-induced interferon. Science. 1984 Jan 13;223(4632):178–181. doi: 10.1126/science.6606850. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Proietti E., Gessani S., Belardelli F., Gresser I. Mouse peritoneal cells confer an antiviral state on mouse cell monolayers: role of interferon. J Virol. 1986 Feb;57(2):456–463. doi: 10.1128/jvi.57.2.456-463.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnitzky D., Yarden A., Zipori D., Kimchi A. Autocrine beta-related interferon controls c-myc suppression and growth arrest during hematopoietic cell differentiation. Cell. 1986 Jul 4;46(1):31–40. doi: 10.1016/0092-8674(86)90857-3. [DOI] [PubMed] [Google Scholar]
- Russell P. H. Newcastle disease virus: the effect of monoclonal antibody in the overlay on virus penetration and the immunoselection of variants. J Gen Virol. 1984 Apr;65(Pt 4):795–798. doi: 10.1099/0022-1317-65-4-795. [DOI] [PubMed] [Google Scholar]
- Schleiffenbaum B., Moser R., Patarroyo M., Fehr J. The cell surface glycoprotein Mac-1 (CD11b/CD18) mediates neutrophil adhesion and modulates degranulation independently of its quantitative cell surface expression. J Immunol. 1989 May 15;142(10):3537–3545. [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Haller O. Interferon-induced Mx protein: a mediator of cellular resistance to influenza virus. Interferon. 1987;8:1–23. [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J. Methods for the purification, assay, characterization and target cell binding of a colony stimulating factor (CSF-1). J Immunol Methods. 1981;42(3):253–284. doi: 10.1016/0022-1759(81)90156-3. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Brown R. E., Kerr I. M. Assay of (2'-5')-oligoadenylic acid synthetase levels in cells and tissues: a convenient poly(I) . poly(C) paper-bound enzyme assay. Methods Enzymol. 1981;79(Pt B):194–199. doi: 10.1016/s0076-6879(81)79029-3. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Gosser L. B., Lockart R. Z., Jr Priming: a nonantiviral function of interferon. J Virol. 1971 Jun;7(6):792–801. doi: 10.1128/jvi.7.6.792-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Balkwill F., Ebsworth N., Rozengurt E. Antiviral and antiproliferative effects of interferons in quiescent fibroblasts are dissociable. Virology. 1985 Dec;147(2):405–412. doi: 10.1016/0042-6822(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Van Heuvel M., Bosveld I. J., Mooren A. A., Trapman J., Zwarthoff E. C. Properties of natural and hybrid murine alpha interferons. J Gen Virol. 1986 Oct;67(Pt 10):2215–2222. doi: 10.1099/0022-1317-67-10-2215. [DOI] [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Szikora J. P., Renauld J. C., Van Roost E., Boon T., Simpson R. J. cDNA cloning of murine interleukin-HP1: homology with human interleukin 6. Eur J Immunol. 1988 Feb;18(2):193–197. doi: 10.1002/eji.1830180202. [DOI] [PubMed] [Google Scholar]
- Zawatzky R., Gresser I., DeMaeyer E., Kirchner H. The role of interferon in the resistance of C57BL/6 mice to various doses of herpes simplex virus type 1. J Infect Dis. 1982 Sep;146(3):405–410. doi: 10.1093/infdis/146.3.405. [DOI] [PubMed] [Google Scholar]
- Zawatzky R., Kirchner H., DeMaeyer-Guignard J., DeMaeyer E. An X-linked locus influences the amount of circulating interferon induced in the mouse by herpes simplex virus type 1. J Gen Virol. 1982 Dec;63(2):325–332. doi: 10.1099/0022-1317-63-2-325. [DOI] [PubMed] [Google Scholar]
- Zwarthoff E. C., Mooren A. T., Trapman J. Organization, structure and expression of murine interferon alpha genes. Nucleic Acids Res. 1985 Feb 11;13(3):791–804. doi: 10.1093/nar/13.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]




