Abstract
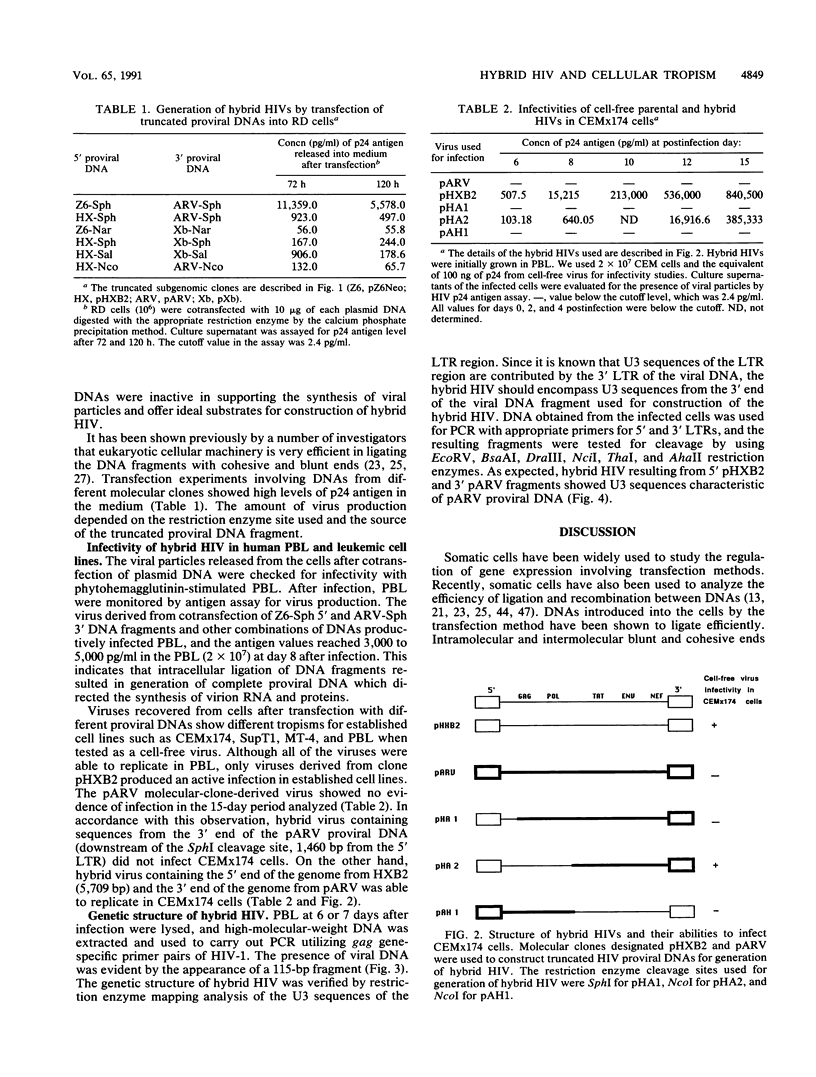
Human immunodeficiency viruses (HIV) isolated from infected individuals show tremendous genetic and biologic diversity. To delineate the genetic determinants underlying specific biologic characteristics, such as rate of replication, cytopathic effects, and ability to infect macrophages and T4 lymphoid cells, generation of hybrid HIV using viruses which exhibit distinct biologic features is essential. To develop methods for generating hybrid HIV, we constructed truncated HIV proviral DNA plasmids. Upon digestion with restriction enzymes, these plasmid DNAs were cotransfected into human rhabdomyosarcoma cells to generate hybrid HIV. The hybrid HIVs derived by this method were infectious upon transmission to both phytohemagglutinin-stimulated peripheral blood lymphocytes and established human leukemic T-cell lines. The virus derived from molecular clone pHXB2 (HIVHTLV-III) productively infected CEMx174 cells. On the other hand, molecular clone pARV (HIVSF2)-derived virus did not show productive infection of CEMx174 cells when used as a cell-free virus. The hybrid HIV containing the 3' end of the genome from pARV and the 5' end of the genome from pHXB2 was effective in infecting CEMx174 cells, but the converse hybrid containing 5' pARV and 3' pHXB2 was not effective in infecting CEMx174 cells. These results suggest that differences in the genes outside of env and nef play a role in the ability of the virus to infect a certain cell type. The intracellular ligation method should be useful in the analysis of related and unrelated HIV-1 isolates with common restriction enzyme cleavage sites.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cann A. J., Karn J. Molecular biology of HIV: new insights into the virus life-cycle. AIDS. 1989;3 (Suppl 1):S19–S34. [PubMed] [Google Scholar]
- Cheng-Mayer C., Quiroga M., Tung J. W., Dina D., Levy J. A. Viral determinants of human immunodeficiency virus type 1 T-cell or macrophage tropism, cytopathogenicity, and CD4 antigen modulation. J Virol. 1990 Sep;64(9):4390–4398. doi: 10.1128/jvi.64.9.4390-4398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Seto D., Levy J. A. Altered host range of HIV-1 after passage through various human cell types. Virology. 1991 Mar;181(1):288–294. doi: 10.1016/0042-6822(91)90494-v. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Seto D., Tateno M., Levy J. A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988 Apr 1;240(4848):80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Genetic variation in AIDS viruses. Cell. 1986 Jul 4;46(1):1–4. doi: 10.1016/0092-8674(86)90851-2. [DOI] [PubMed] [Google Scholar]
- De B. K., Srinivasan A. Detection of human immunodeficiency virus (HIV) and human lymphotropic virus (HTLV) type I or II dual infections by polymerase chain reaction. Oncogene. 1989 Dec;4(12):1533–1535. [PubMed] [Google Scholar]
- Etkin A. F., Bukrinsky M. I. Heterogeneity of the ARV-2 strain and natural isolates of the human immunodeficiency virus. Arch Virol. 1989;108(3-4):271–277. doi: 10.1007/BF01310939. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fenyö E. M., Albert J., Asjö B. Replicative capacity, cytopathic effect and cell tropism of HIV. AIDS. 1989;3 (Suppl 1):S5–12. doi: 10.1097/00002030-198901001-00002. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Collalti E., Ratner L., Gallo R. C., Wong-Staal F. A molecular clone of HTLV-III with biological activity. Nature. 1985 Jul 18;316(6025):262–265. doi: 10.1038/316262a0. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Ensoli B., Ivanoff L., Chamberlain M., Petteway S., Ratner L., Gallo R. C., Wong-Staal F. The sor gene of HIV-1 is required for efficient virus transmission in vitro. Science. 1987 Aug 21;237(4817):888–893. doi: 10.1126/science.3497453. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Ensoli B., Looney D., Rose A., Gallo R. C., Saag M. S., Shaw G. M., Hahn B. H., Wong-Staal F. Biologically diverse molecular variants within a single HIV-1 isolate. Nature. 1988 Aug 4;334(6181):444–447. doi: 10.1038/334444a0. [DOI] [PubMed] [Google Scholar]
- Folger K. R., Wong E. A., Wahl G., Capecchi M. R. Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol. 1982 Nov;2(11):1372–1387. doi: 10.1128/mcb.2.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Golub E. I., Li G. G., Volsky D. J. Differences in the basal activity of the long terminal repeat determine different replicative capacities of two closely related human immunodeficiency virus type 1 isolates. J Virol. 1990 Aug;64(8):3654–3660. doi: 10.1128/jvi.64.8.3654-3660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit J., de Wolf F., Paul D. A., Epstein L. G., Lange J. M., Krone W. J., Speelman H., Wolters E. C., Van der Noordaa J., Oleske J. M. Expression of human immunodeficiency virus antigen (HIV-Ag) in serum and cerebrospinal fluid during acute and chronic infection. Lancet. 1986 Jul 26;2(8500):177–180. doi: 10.1016/s0140-6736(86)92485-2. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Taylor M. E., Redfield R. R., Markham P. D., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Parks E. S., Parks W. P. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science. 1986 Jun 20;232(4757):1548–1553. doi: 10.1126/science.3012778. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A. Development of antiviral drugs for the treatment of AIDS: strategies and prospects. J Acquir Immune Defic Syndr. 1989;2(4):311–334. [PubMed] [Google Scholar]
- Hirsch I., Spire B., Tsunetsugu-Yokota Y., Neuveut C., Sire J., Chermann J. C. Differences in replication and cytopathogenicity of human immunodeficiency virus type 1 (HIV-1) are not determined by long terminal repeats (LTR). Virology. 1990 Aug;177(2):759–763. doi: 10.1016/0042-6822(90)90544-2. [DOI] [PubMed] [Google Scholar]
- Hoy C. A., Fuscoe J. C., Thompson L. H. Recombination and ligation of transfected DNA in CHO mutant EM9, which has high levels of sister chromatid exchange. Mol Cell Biol. 1987 May;7(5):2007–2011. doi: 10.1128/mcb.7.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman S., Jannoun-Nasr R., York D., Luciw P. A., Robinson R., Srinivasan A. Homologous recombination between human immunodeficiency viral DNAs in cultured human cells: analysis of the factors influencing recombination. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1051–1060. doi: 10.1016/s0006-291x(88)80981-1. [DOI] [PubMed] [Google Scholar]
- Kopchick J. J., Stacey D. W. Differences in intracellular DNA ligation after microinjection and transfection. Mol Cell Biol. 1984 Feb;4(2):240–246. doi: 10.1128/mcb.4.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Cheng-Mayer C., Dina D., Luciw P. A. AIDS retrovirus (ARV-2) clone replicates in transfected human and animal fibroblasts. Science. 1986 May 23;232(4753):998–1001. doi: 10.1126/science.3010461. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Miller C. K., Temin H. M. High-efficiency ligation and recombination of DNA fragments by vertebrate cells. Science. 1983 May 6;220(4597):606–609. doi: 10.1126/science.6301012. [DOI] [PubMed] [Google Scholar]
- Mims C. A. The pathogenetic basis of viral tropism. Am J Pathol. 1989 Sep;135(3):447–455. [PMC free article] [PubMed] [Google Scholar]
- Pomerantz R. J., Feinberg M. B., Andino R., Baltimore D. The long terminal repeat is not a major determinant of the cellular tropism of human immunodeficiency virus type 1. J Virol. 1991 Feb;65(2):1041–1045. doi: 10.1128/jvi.65.2.1041-1045.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. The immunopathogenesis of HIV infection. Adv Immunol. 1989;47:377–431. doi: 10.1016/s0065-2776(08)60665-3. [DOI] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991 Jan 10;349(6305):167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- Srinivasan A., Anand R., York D., Ranganathan P., Feorino P., Schochetman G., Curran J., Kalyanaraman V. S., Luciw P. A., Sanchez-Pescador R. Molecular characterization of human immunodeficiency virus from Zaire: nucleotide sequence analysis identifies conserved and variable domains in the envelope gene. Gene. 1987;52(1):71–82. doi: 10.1016/0378-1119(87)90396-9. [DOI] [PubMed] [Google Scholar]
- Srinivasan A., Goldsmith C. S., York D., Anand R., Luciw P., Schochetman G., Palmer E., Bohan C. Studies on human immunodeficiency virus-induced cytopathic effects: use of human rhabdomyosarcoma (RD) cells. Arch Virol. 1988;99(1-2):21–30. doi: 10.1007/BF01311020. [DOI] [PubMed] [Google Scholar]
- Srinivasan A., Reddy E. P., Aaronson S. A. Abelson murine leukemia virus: molecular cloning of infectious integrated proviral DNA. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2077–2081. doi: 10.1073/pnas.78.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A., York D., Butler D., Jr, Jannoun-Nasr R., Getchell J., McCormick J., Ou C. Y., Myers G., Smith T., Chen E. Molecular characterization of HIV-1 isolated from a serum collected in 1976: nucleotide sequence comparison to recent isolates and generation of hybrid HIV. AIDS Res Hum Retroviruses. 1989 Apr;5(2):121–129. doi: 10.1089/aid.1989.5.121. [DOI] [PubMed] [Google Scholar]
- Srinivasan A., York D., Jannoun-Nasr R., Kalyanaraman S., Swan D., Benson J., Bohan C., Luciw P. A., Schnoll S., Robinson R. A. Generation of hybrid human immunodeficiency virus by homologous recombination. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6388–6392. doi: 10.1073/pnas.86.16.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer D. A., Holland J. J. Rapid evolution of RNA viruses. Annu Rev Microbiol. 1987;41:409–433. doi: 10.1146/annurev.mi.41.100187.002205. [DOI] [PubMed] [Google Scholar]
- Strebel K., Daugherty D., Clouse K., Cohen D., Folks T., Martin M. A. The HIV 'A' (sor) gene product is essential for virus infectivity. Nature. 1987 Aug 20;328(6132):728–730. doi: 10.1038/328728a0. [DOI] [PubMed] [Google Scholar]
- Tersmette M., Gruters R. A., de Wolf F., de Goede R. E., Lange J. M., Schellekens P. T., Goudsmit J., Huisman H. G., Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989 May;63(5):2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upcroft P., Carter B., Kidson C. Mammalian cell function mediating recombination of genetic elements. Nucleic Acids Res. 1980 Dec 11;8(23):5835–5844. doi: 10.1093/nar/8.23.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S. HIV genome variability in vivo. AIDS. 1989;3 (Suppl 1):S13–S18. doi: 10.1097/00002030-198901001-00003. [DOI] [PubMed] [Google Scholar]
- Wake C. T., Gudewicz T., Porter T., White A., Wilson J. H. How damaged is the biologically active subpopulation of transfected DNA? Mol Cell Biol. 1984 Mar;4(3):387–398. doi: 10.1128/mcb.4.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. H., Berget P. B., Pipas J. M. Somatic cells efficiently join unrelated DNA segments end-to-end. Mol Cell Biol. 1982 Oct;2(10):1258–1269. doi: 10.1128/mcb.2.10.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York-Higgins D., Cheng-Mayer C., Bauer D., Levy J. A., Dina D. Human immunodeficiency virus type 1 cellular host range, replication, and cytopathicity are linked to the envelope region of the viral genome. J Virol. 1990 Aug;64(8):4016–4020. doi: 10.1128/jvi.64.8.4016-4020.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourno J., Fisher A. G., Looney D. J., Gallo R. C., Wong-Staal F. A recombinant clone of HIV-1 preferentially transmitted in normal peripheral blood mononuclear cells. AIDS Res Hum Retroviruses. 1989 Dec;5(6):565–575. doi: 10.1089/aid.1989.5.565. [DOI] [PubMed] [Google Scholar]




