Abstract
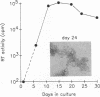
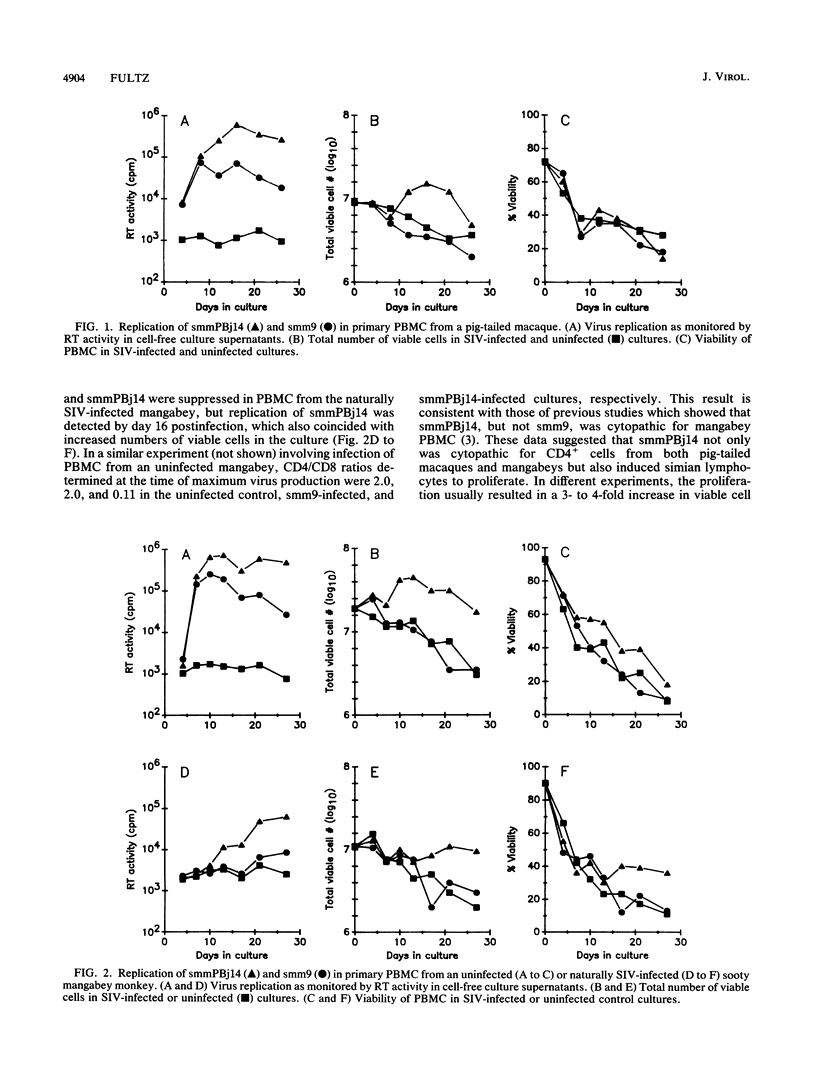
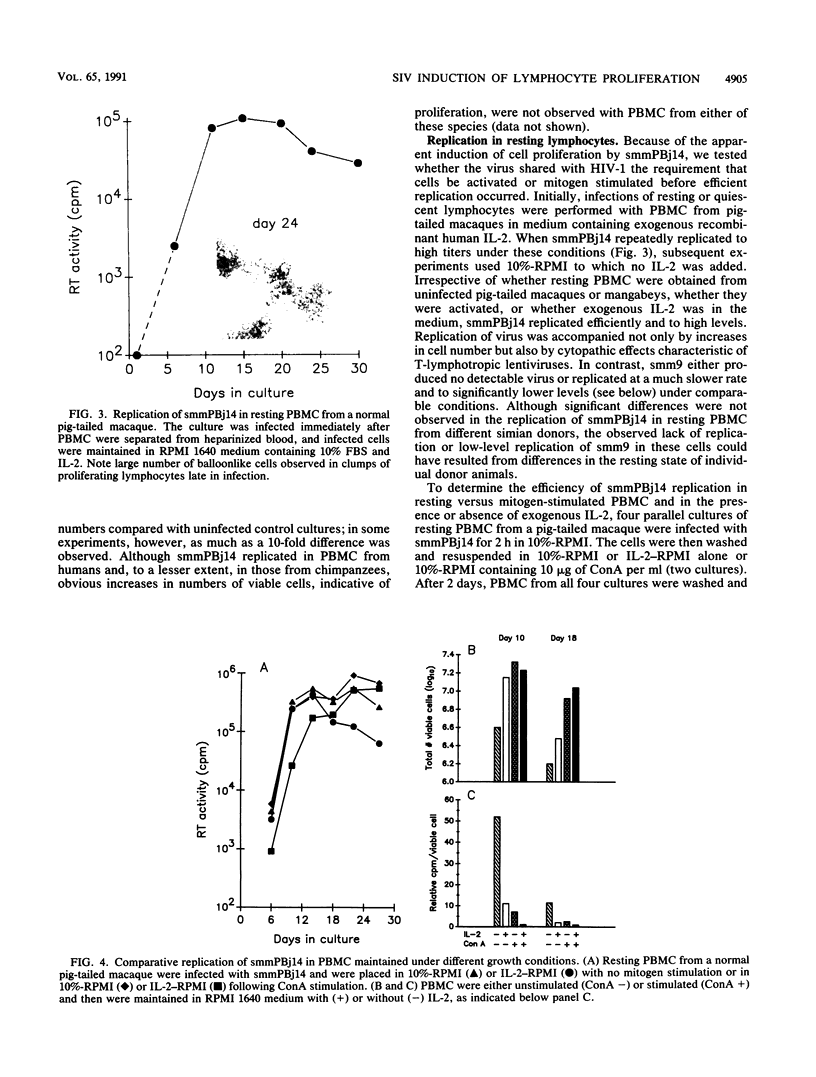
A variant of simian immunodeficiency virus from sooty mangabey monkeys (SIVsmm), termed SIVsmmPBj14, was previously identified and shown to induce acute disease and death within 1 to 2 weeks of inoculation of pig-tailed macaques and mangabey monkeys (P. N. Fultz, H. M. McClure, D. C. Anderson, and W. M. Switzer, AIDS Res. Hum. Retroviruses 5:397-409, 1989). SIVsmmPBj14 differed from its parent virus, SIVsmm9, not only in pathogenicity but also in multiple in vitro properties. As a first approach to understanding the biological and molecular mechanisms responsible for the acute disease and death induced by this variant, virus-host cell interactions of SIVsmmPBj14 and SIVsmm9 were studied. Initial rates of replication of the two viruses were identical in primary peripheral blood mononuclear cells (PBMC) from normal pig-tailed macaques and mangabey monkeys, but SIVsmmPBj14 infection always resulted in higher yields of virus than did SIVsmm9 infection, as assessed by levels of reverse transcriptase activity in culture supernatants. Surprisingly, despite its cytopathicity for macaque and mangabey CD4+ cells, replication of SIVsmmPBj14 was accompanied by up to 10-fold increases in number of viable cells compared with cell numbers in uninfected or SIVsmm9-infected cultures. Furthermore, SIVsmmPBj14 was shown to infect and replicate in resting PBMC just as efficiently as in mitogen-stimulated PBMC, irrespective of whether exogenous interleukin-2 (IL-2) or antibodies that neutralized IL-2 were added to culture media. Accumulation of virus in culture supernatants of resting PBMC preceded by several days the appearance of activated cells which expressed the IL-2 receptor alpha subunit (CD25), suggesting that activation of cells was not essential for replication. The ability to activate and to induce simian PBMC to proliferate appeared specific for the acutely lethal variant because incorporation of [3H]thymidine by PBMC from naive animals was observed only upon incubation with concentrated, heat-inactivated SIVsmmPBj14 and not with other viruses. Both CD4(+)- and CD8(+)-enriched cell populations proliferated in response to SIVsmmPBj14. These results are consistent with in vivo observations and suggest that the abilities both to replicate in resting cells and to induce lymphocytes to proliferate may contribute to the extreme virulence of SIVsmmPBj14.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chirmule N., Kalyanaraman V. S., Saxinger C., Wong-Staal F., Ghrayeb J., Pahwa S. Localization of B-cell stimulatory activity of HIV-1 to the carboxyl terminus of gp41. AIDS Res Hum Retroviruses. 1990 Mar;6(3):299–305. doi: 10.1089/aid.1990.6.299. [DOI] [PubMed] [Google Scholar]
- Coombs R. W., Collier A. C., Allain J. P., Nikora B., Leuther M., Gjerset G. F., Corey L. Plasma viremia in human immunodeficiency virus infection. N Engl J Med. 1989 Dec 14;321(24):1626–1631. doi: 10.1056/NEJM198912143212402. [DOI] [PubMed] [Google Scholar]
- Dewhurst S., Embretson J. E., Anderson D. C., Mullins J. I., Fultz P. N. Sequence analysis and acute pathogenicity of molecularly cloned SIVSMM-PBj14. Nature. 1990 Jun 14;345(6276):636–640. doi: 10.1038/345636a0. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Anderson D. C., Switzer W. M. Identification and biologic characterization of an acutely lethal variant of simian immunodeficiency virus from sooty mangabeys (SIV/SMM). AIDS Res Hum Retroviruses. 1989 Aug;5(4):397–409. doi: 10.1089/aid.1989.5.397. [DOI] [PubMed] [Google Scholar]
- Fultz P. N., Stricker R. B., McClure H. M., Anderson D. C., Switzer W. M., Horaist C. Humoral response to SIV/SMM infection in macaque and mangabey monkeys. J Acquir Immune Defic Syndr. 1990;3(4):319–329. [PubMed] [Google Scholar]
- Gazzolo L., Duc Dodon M. Direct activation of resting T lymphocytes by human T-lymphotropic virus type I. Nature. 1987 Apr 16;326(6114):714–717. doi: 10.1038/326714a0. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Kennedy P. G., Ghotbi Z., Clements J. E., Stanley J., Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986 Apr;58(1):67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., Moudgil T., Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989 Dec 14;321(24):1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- Hofmann B., Nishanian P., Baldwin R. L., Insixiengmay P., Nel A., Fahey J. L. HIV inhibits the early steps of lymphocyte activation, including initiation of inositol phospholipid metabolism. J Immunol. 1990 Dec 1;145(11):3699–3705. [PubMed] [Google Scholar]
- Holsti M. A., Raulet D. H. IL-6 and IL-1 synergize to stimulate IL-2 production and proliferation of peripheral T cells. J Immunol. 1989 Oct 15;143(8):2514–2519. [PubMed] [Google Scholar]
- Honda M., Kitamura K., Mizutani Y., Oishi M., Arai M., Okura T., Igarahi K., Yasukawa K., Hirano T., Kishimoto T. Quantitative analysis of serum IL-6 and its correlation with increased levels of serum IL-2R in HIV-induced diseases. J Immunol. 1990 Dec 15;145(12):4059–4064. [PubMed] [Google Scholar]
- Horak I. D., Popovic M., Horak E. M., Lucas P. J., Gress R. E., June C. H., Bolen J. B. No T-cell tyrosine protein kinase signalling or calcium mobilization after CD4 association with HIV-1 or HIV-1 gp120. Nature. 1990 Dec 6;348(6301):557–560. doi: 10.1038/348557a0. [DOI] [PubMed] [Google Scholar]
- Houssiau F. A., Coulie P. G., Van Snick J. Distinct roles of IL-1 and IL-6 in human T cell activation. J Immunol. 1989 Oct 15;143(8):2520–2524. [PubMed] [Google Scholar]
- Kornfeld H., Cruikshank W. W., Pyle S. W., Berman J. S., Center D. M. Lymphocyte activation by HIV-1 envelope glycoprotein. Nature. 1988 Sep 29;335(6189):445–448. doi: 10.1038/335445a0. [DOI] [PubMed] [Google Scholar]
- Laing T. J., Weiss A. Evidence for IL-2 independent proliferation in human T cells. J Immunol. 1988 Feb 15;140(4):1056–1062. [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- McClure H. M., Anderson D. C., Fultz P. N., Ansari A. A., Lockwood E., Brodie A. Spectrum of disease in macaque monkeys chronically infected with SIV/SMM. Vet Immunol Immunopathol. 1989 May;21(1):13–24. doi: 10.1016/0165-2427(89)90126-8. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- McGrath M., Witte O., Pincus T., Weissman I. L. Retrovirus purification: method that conserves envelope glycoprotein and maximizes infectivity. J Virol. 1978 Mar;25(3):923–927. doi: 10.1128/jvi.25.3.923-927.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz M., Rapaport R., Oleske J. M., Connor E. M., Koenigsberger M. R., Denny T., Epstein L. G. Elevated serum levels of tumor necrosis factor are associated with progressive encephalopathy in children with acquired immunodeficiency syndrome. Am J Dis Child. 1989 Jul;143(7):771–774. doi: 10.1001/archpedi.1989.02150190021012. [DOI] [PubMed] [Google Scholar]
- Moldwin R. L., Lancki D. W., Herold K. C., Fitch F. W. An antigen receptor-driven, interleukin 2-independent pathway for proliferation of murine cytolytic T lymphocyte clones. J Exp Med. 1986 Jun 1;163(6):1566–1582. doi: 10.1084/jem.163.6.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Bond M. W., Coffman R. L., Ohara J., Paul W. E. T-cell and mast cell lines respond to B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5654–5658. doi: 10.1073/pnas.83.15.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair M. P., Pottathil R., Heimer E. P., Schwartz S. A. Immunoregulatory activities of human immunodeficiency virus (HIV) proteins: effect of HIV recombinant and synthetic peptides on immunoglobulin synthesis and proliferative responses by normal lymphocytes. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6498–6502. doi: 10.1073/pnas.85.17.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa S., Pahwa R., Saxinger C., Gallo R. C., Good R. A. Influence of the human T-lymphotropic virus/lymphadenopathy-associated virus on functions of human lymphocytes: evidence for immunosuppressive effects and polyclonal B-cell activation by banded viral preparations. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8198–8202. doi: 10.1073/pnas.82.23.8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauza C. D., Galindo J., Richman D. D. Human immunodeficiency virus infection of monoblastoid cells: cellular differentiation determines the pattern of virus replication. J Virol. 1988 Oct;62(10):3558–3564. doi: 10.1128/jvi.62.10.3558-3564.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Bressler P., Kinter A., Duh E., Timmer W. C., Rabson A., Justement J. S., Stanley S., Fauci A. S. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990 Jul 1;172(1):151–158. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Hennighausen L., Siebenlist U. Inducible nuclear factor binding to the kappa B elements of the human immunodeficiency virus enhancer in T cells can be blocked by cyclosporin A in a signal-dependent manner. J Virol. 1990 Aug;64(8):4037–4041. doi: 10.1128/jvi.64.8.4037-4041.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittman S. M., Greenhouse J. J., Psallidopoulos M. C., Baseler M., Salzman N. P., Fauci A. S., Lane H. C. Increasing viral burden in CD4+ T cells from patients with human immunodeficiency virus (HIV) infection reflects rapidly progressive immunosuppression and clinical disease. Ann Intern Med. 1990 Sep 15;113(6):438–443. doi: 10.7326/0003-4819-113-6-438. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Lane H. C., Higgins S. E., Folks T., Fauci A. S. Direct polyclonal activation of human B lymphocytes by the acquired immune deficiency syndrome virus. Science. 1986 Sep 5;233(4768):1084–1086. doi: 10.1126/science.3016902. [DOI] [PubMed] [Google Scholar]
- Stevenson M., Stanwick T. L., Dempsey M. P., Lamonica C. A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990 May;9(5):1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong-Starksen S. E., Welsh T. M., Peterlin B. M. Differences in transcriptional enhancers of HIV-1 and HIV-2. Response to T cell activation signals. J Immunol. 1990 Dec 15;145(12):4348–4354. [PubMed] [Google Scholar]
- Vyakarnam A., McKeating J., Meager A., Beverley P. C. Tumour necrosis factors (alpha, beta) induced by HIV-1 in peripheral blood mononuclear cells potentiate virus replication. AIDS. 1990 Jan;4(1):21–27. doi: 10.1097/00002030-199001000-00003. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- Zagury D., Bernard J., Leonard R., Cheynier R., Feldman M., Sarin P. S., Gallo R. C. Long-term cultures of HTLV-III--infected T cells: a model of cytopathology of T-cell depletion in AIDS. Science. 1986 Feb 21;231(4740):850–853. doi: 10.1126/science.2418502. [DOI] [PubMed] [Google Scholar]
- Zhang J. Y., Martin L. N., Watson E. A., Montelaro R. C., West M., Epstein L., Murphey-Corb M. Simian immunodeficiency virus/delta-induced immunodeficiency disease in rhesus monkeys: relation of antibody response and antigenemia. J Infect Dis. 1988 Dec;158(6):1277–1286. doi: 10.1093/infdis/158.6.1277. [DOI] [PubMed] [Google Scholar]