Abstract
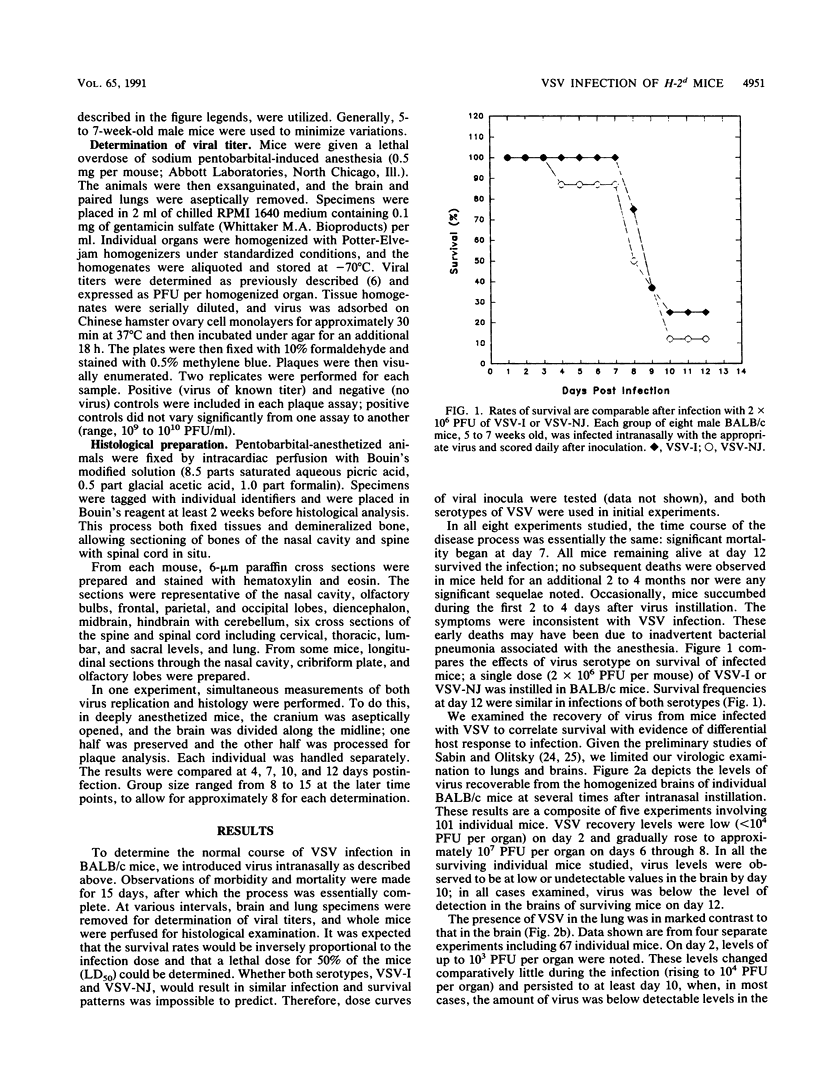
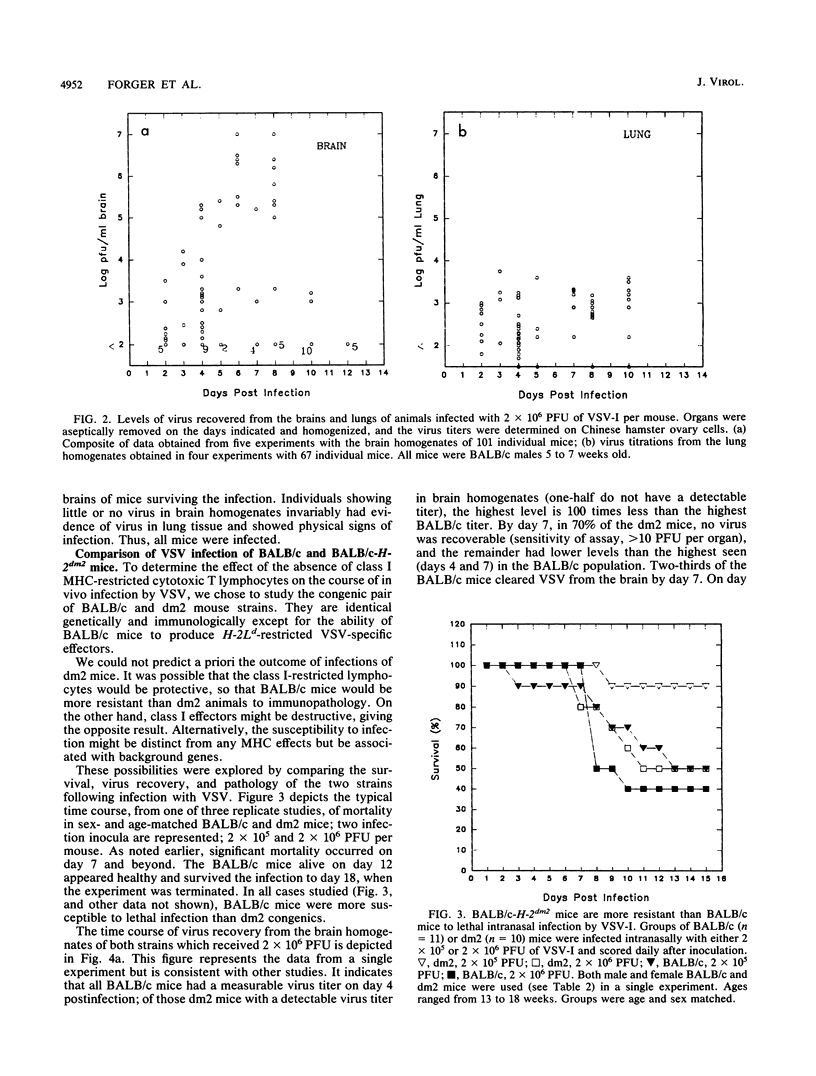
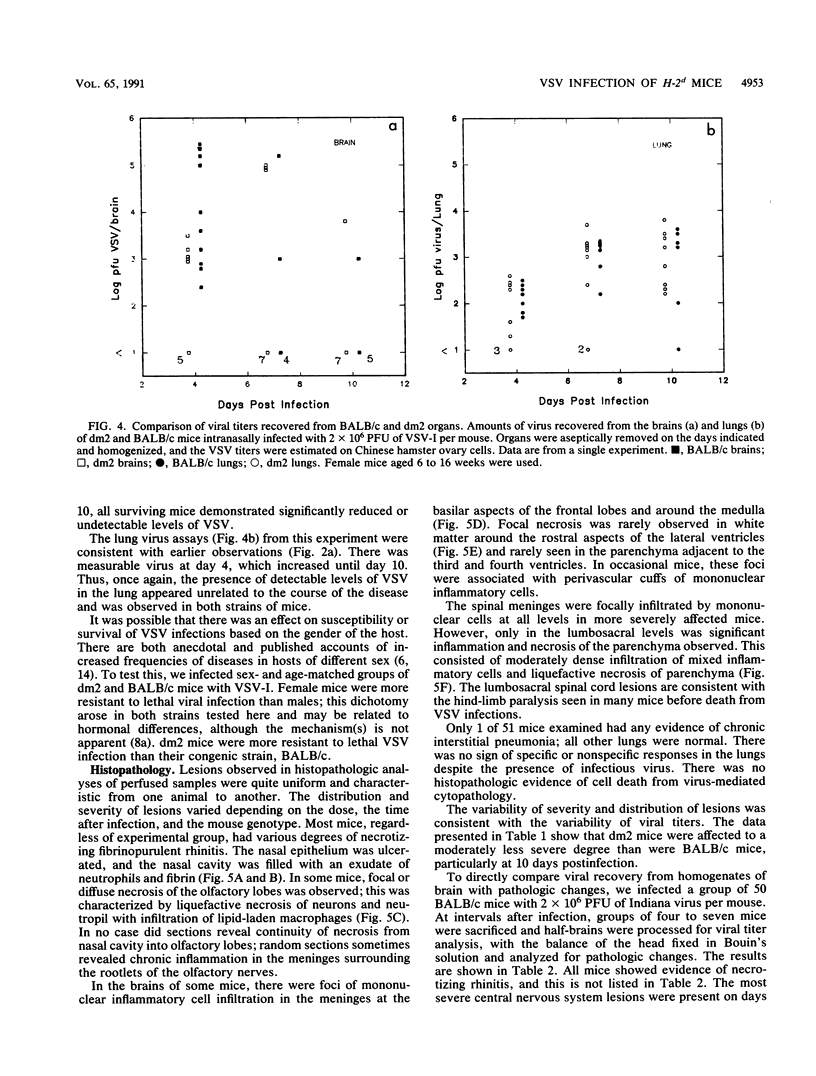
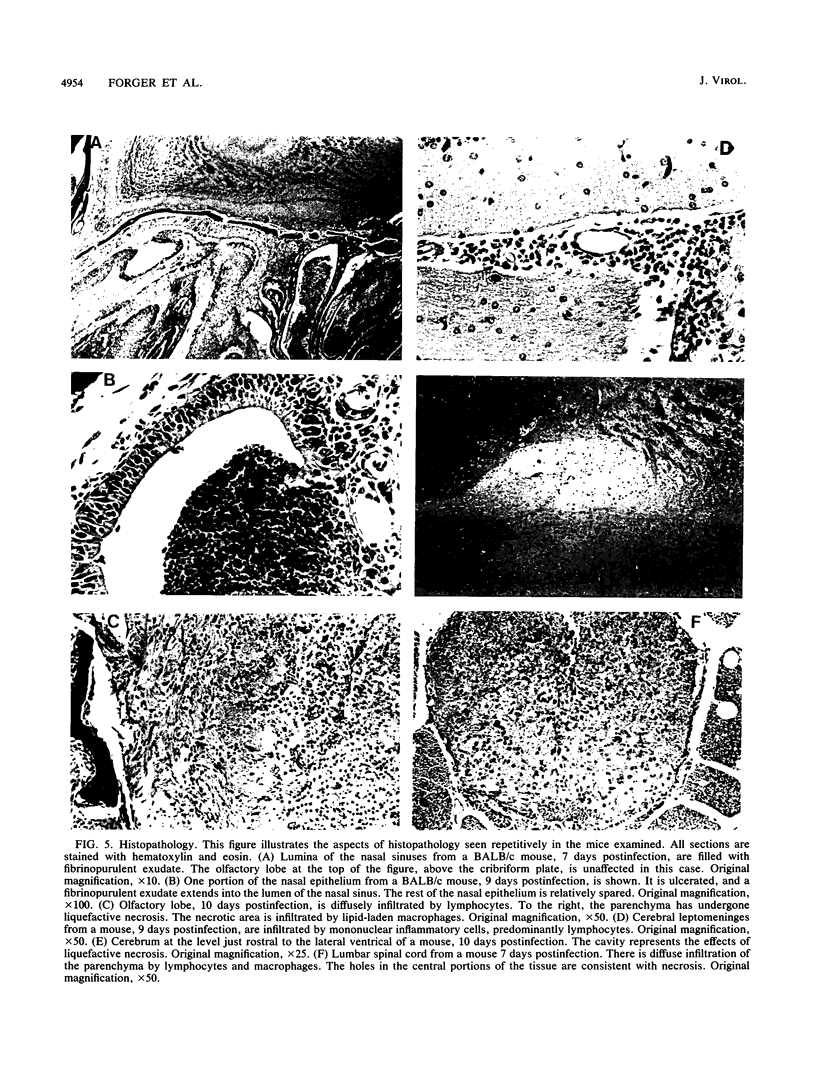
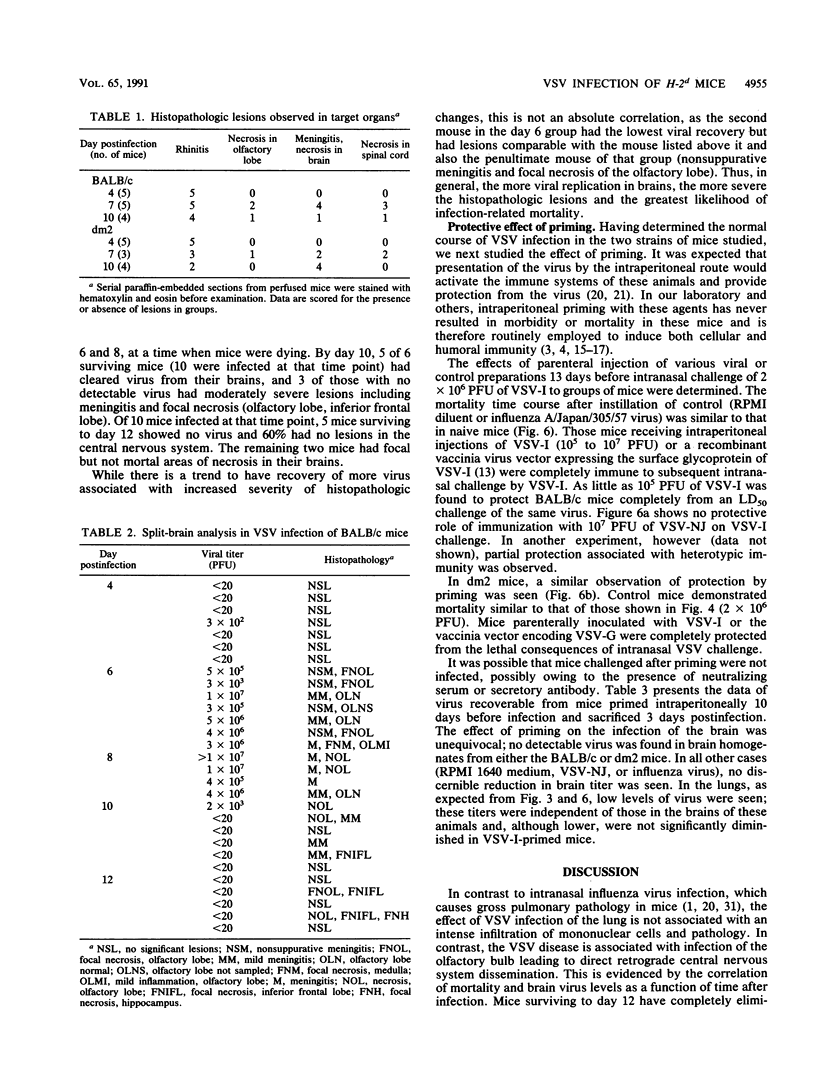
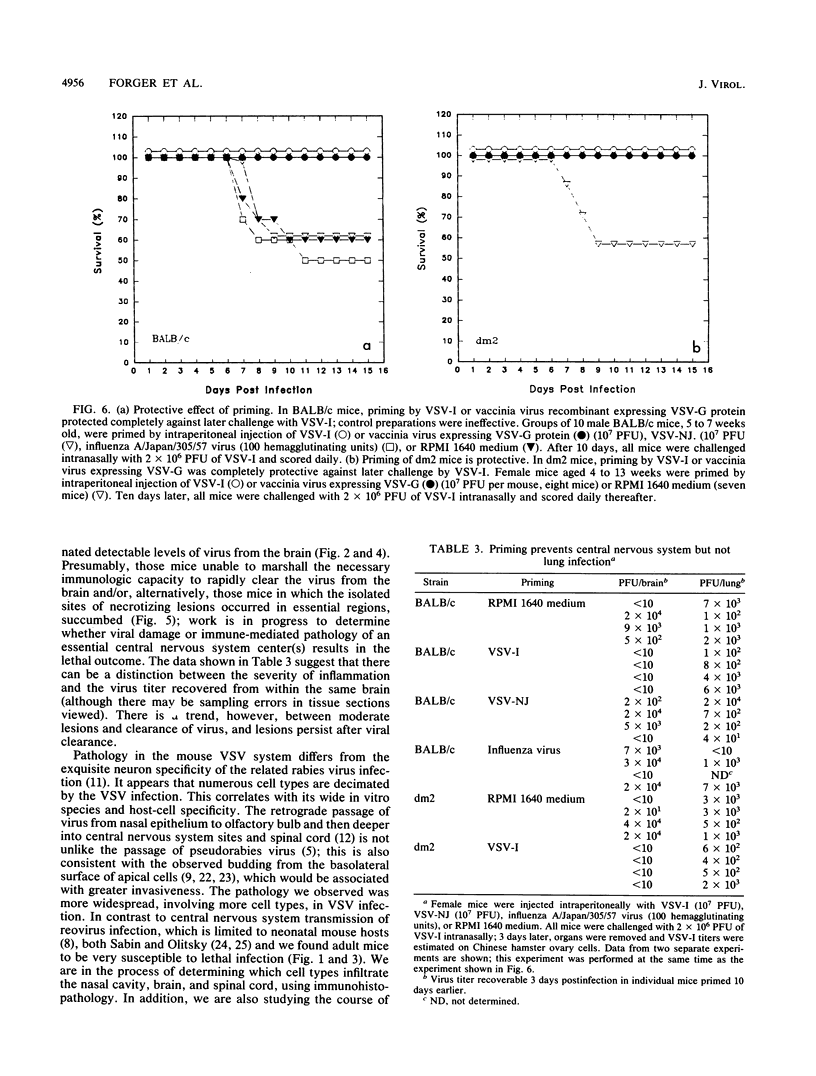
BALB/c mice and congenic H-2Ld-deficient BALB/c-H-2dm2 (dm2) mice were experimentally infected intranasally with isolates of vesicular stomatitis virus (VSV). The survival of infected hosts, viral replication in lungs and brains, and histopathologic in the two mouse strains were compared. In both strains of mice, mortality occurred during the period 7 to 10 days postinfection. However, dm2 mice were relatively resistant to lethal infections. Viral replication occurred at low levels in the lungs of both strains and did not evoke significant pathologic changes. In contrast, viral replication in the brains was much greater; in the BALB/c strain, this was accompanied by more frequent and more severe pathologic changes. In general, mice surviving at day 10 had effectively cleared virus from central nervous system but not respiratory sites. Evidence is presented that viral replication occurs first in the nasal cavity and is transmitted both to the lungs and to the olfactory bulb where focal cytopathology occurs. Virus enters the ventricles, causing encephalitis; necrosis occurs around the ventricles and in the lumbosacral region of the spinal cord. Necrotic lesions were accompanied by mononuclear infiltration. Mice immunized with virus of the same serotype or with a vaccinia virus hybrid encoding the VSV glycoprotein were protected from lethal infection; in contrast, mice immunized with heterotypic virus were susceptible to challenge.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennink J. R., Yewdell J. W. Recombinant vaccinia viruses as vectors for studying T lymphocyte specificity and function. Curr Top Microbiol Immunol. 1990;163:153–184. doi: 10.1007/978-3-642-75605-4_6. [DOI] [PubMed] [Google Scholar]
- Bennink J., Effros R. B., Doherty P. C. Influenzal pneumonia: early appearance of cross-reactive T cells in lungs of mice primed with heterologous type A viruses. Immunology. 1978 Sep;35(3):503–509. [PMC free article] [PubMed] [Google Scholar]
- Browning M. J., Huang A. S., Reiss C. S. Cytolytic T lymphocytes from the BALB/c-H-2dm2 mouse recognize the vesicular stomatitis virus glycoprotein and are restricted by class II MHC antigens. J Immunol. 1990 Aug 1;145(3):985–994. [PubMed] [Google Scholar]
- Card J. P., Rinaman L., Schwaber J. S., Miselis R. R., Whealy M. E., Robbins A. K., Enquist L. W. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990 Jun;10(6):1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave D. R., Hendrickson F. M., Huang A. S. Defective interfering virus particles modulate virulence. J Virol. 1985 Aug;55(2):366–373. doi: 10.1128/jvi.55.2.366-373.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciavarra R., Forman J. H-2L-restricted recognition of viral antigens In the H-2d haplotype, anti-vesicular stomatitis virus cytotoxic T cells are restricted solely by H-2L. J Exp Med. 1982 Sep 1;156(3):778–790. doi: 10.1084/jem.156.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand A., Gagner J. P., Morrison L. A., Fields B. N. Penetration of the nervous systems of suckling mice by mammalian reoviruses. J Virol. 1991 Jan;65(1):123–131. doi: 10.1128/jvi.65.1.123-131.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S., von Bonsdorff C. H., Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984 Aug;38(1):65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Rose J. K. Nucleotide sequence of a cDNA clone encoding the entire glycoprotein from the New Jersey serotype of vesicular stomatitis virus. J Virol. 1983 Apr;46(1):162–169. doi: 10.1128/jvi.46.1.162-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. C. Biological basis of rabies virus neurovirulence in mice: comparative pathogenesis study using the immunoperoxidase technique. J Virol. 1991 Jan;65(1):537–540. doi: 10.1128/jvi.65.1.537-540.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh B., Kristensson K., Norrby E. Selective infections of olfactory and respiratory epithelium by vesicular stomatitis and Sendai viruses. Neuropathol Appl Neurobiol. 1987 Mar-Apr;13(2):111–122. doi: 10.1111/j.1365-2990.1987.tb00175.x. [DOI] [PubMed] [Google Scholar]
- Petrarca M. A., Reiss C. S., Diamond D. C., Boni J., Burakoff S. J., Faller D. V. T cell hybridomas define the class II MHC-restricted response to vesicular stomatitis virus infection. Microb Pathog. 1988 Nov;5(5):319–332. doi: 10.1016/0882-4010(88)90033-2. [DOI] [PubMed] [Google Scholar]
- Reiss C. S., Chen S. S., Huang A. S., Doherty R. VSV G protein induces murine cytolytic T lymphocytes. Microb Pathog. 1986 Jun;1(3):261–267. doi: 10.1016/0882-4010(86)90050-1. [DOI] [PubMed] [Google Scholar]
- Reiss C. S., Evans G. A., Margulies D. H., Seidman J. G., Burakoff S. J. Allospecific and virus-specific cytolytic T lymphocytes are restricted to the N or C1 domain of H-2 antigens expressed on L cells after DNA-mediated gene transfer. Proc Natl Acad Sci U S A. 1983 May;80(9):2709–2712. doi: 10.1073/pnas.80.9.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss C. S., Liu L. L., Mowshowitz S. L. Interferon production by cultured murine splenocytes in response to influenza virus-infected cells. J Interferon Res. 1984;4(1):81–89. doi: 10.1089/jir.1984.4.81. [DOI] [PubMed] [Google Scholar]
- Reiss C. S., Schulman J. L. Cellular immune responses of mice to influenza virus infection. Cell Immunol. 1980 Dec;56(2):502–509. doi: 10.1016/0008-8749(80)90124-0. [DOI] [PubMed] [Google Scholar]
- Reiss C. S., Schulman J. L. Cellular immune responses of mice to influenza virus vaccines. J Immunol. 1980 Nov;125(5):2182–2188. [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Fitzpatrick J. P., Compans R. W. Polarity of influenza and vesicular stomatitis virus maturation in MDCK cells: lack of a requirement for glycosylation of viral glycoproteins. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6430–6434. doi: 10.1073/pnas.76.12.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULMAN J. L., KILBOURNE E. D. INDUCTION OF PARTIAL SPECIFIC HETEROTYPIC IMMUNITY IN MICE BY A SINGLE INFECTION WITH INFLUENZA A VIRUS. J Bacteriol. 1965 Jan;89:170–174. doi: 10.1128/jb.89.1.170-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan D., Sun H., Lindahl K. F., Meyer E., Hämmerling G., Hood L., Steinmetz M. Organization and evolution of D region class I genes in the mouse major histocompatibility complex. J Exp Med. 1986 May 1;163(5):1227–1244. doi: 10.1084/jem.163.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepol S. B., Lefrancois L., Holland J. J. Sequences of the major antibody binding epitopes of the Indiana serotype of vesicular stomatitis virus. Virology. 1986 Jan 30;148(2):312–325. doi: 10.1016/0042-6822(86)90328-4. [DOI] [PubMed] [Google Scholar]
- Yap K. L., Braciale T. J., Ada G. L. Role of T-cell function in recovery from murine influenza infection. Cell Immunol. 1979 Mar 15;43(2):341–351. doi: 10.1016/0008-8749(79)90178-3. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Mackett M., Lefrancois L., Lyles D. S., Moss B. Recognition of cloned vesicular stomatitis virus internal and external gene products by cytotoxic T lymphocytes. J Exp Med. 1986 Jun 1;163(6):1529–1538. doi: 10.1084/jem.163.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]




