Abstract
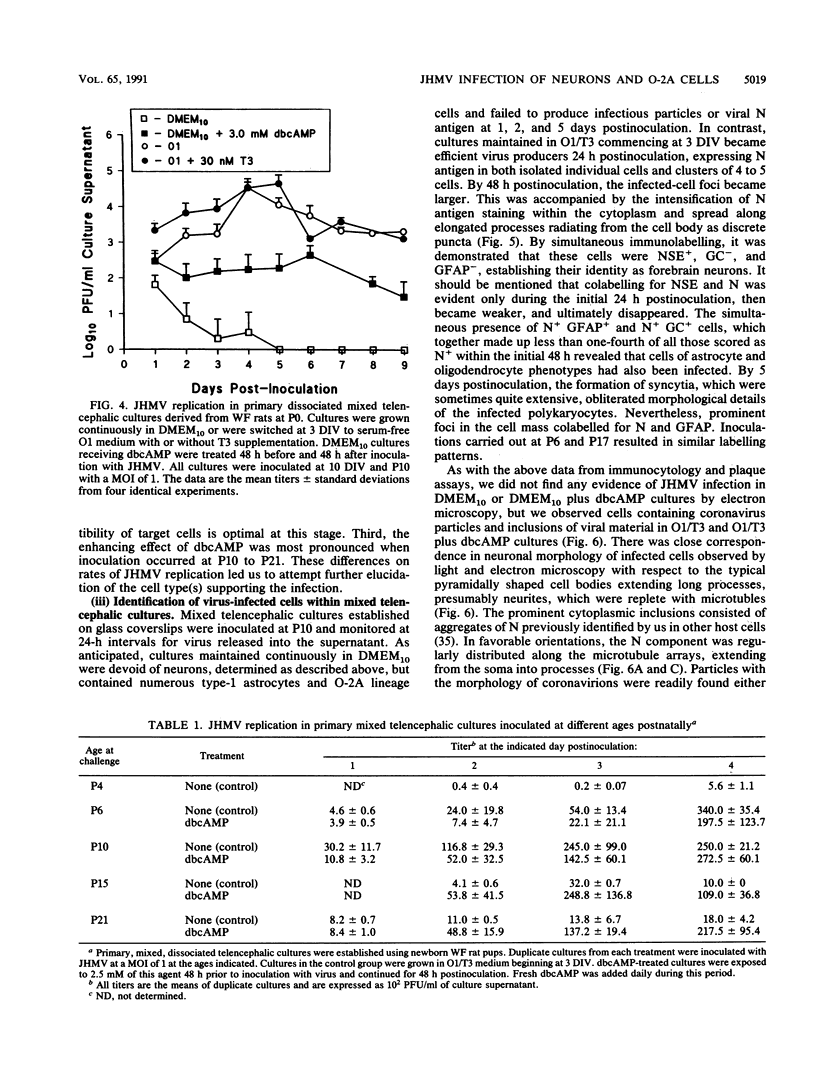
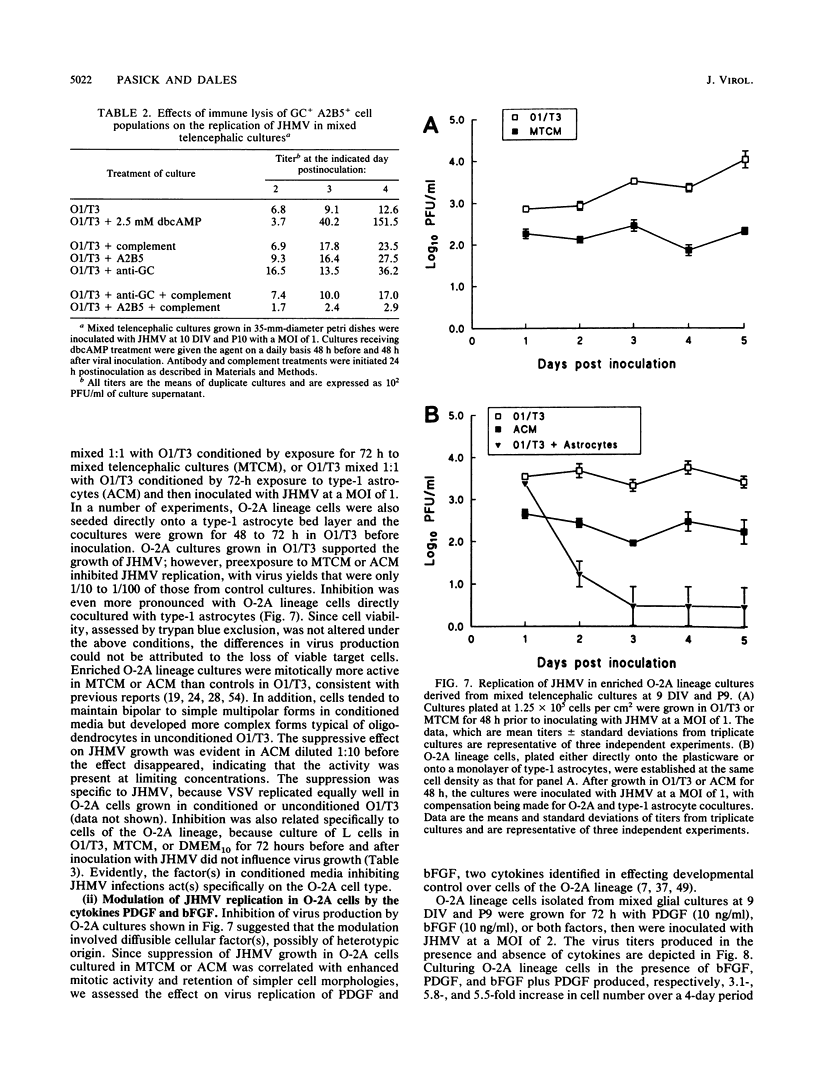
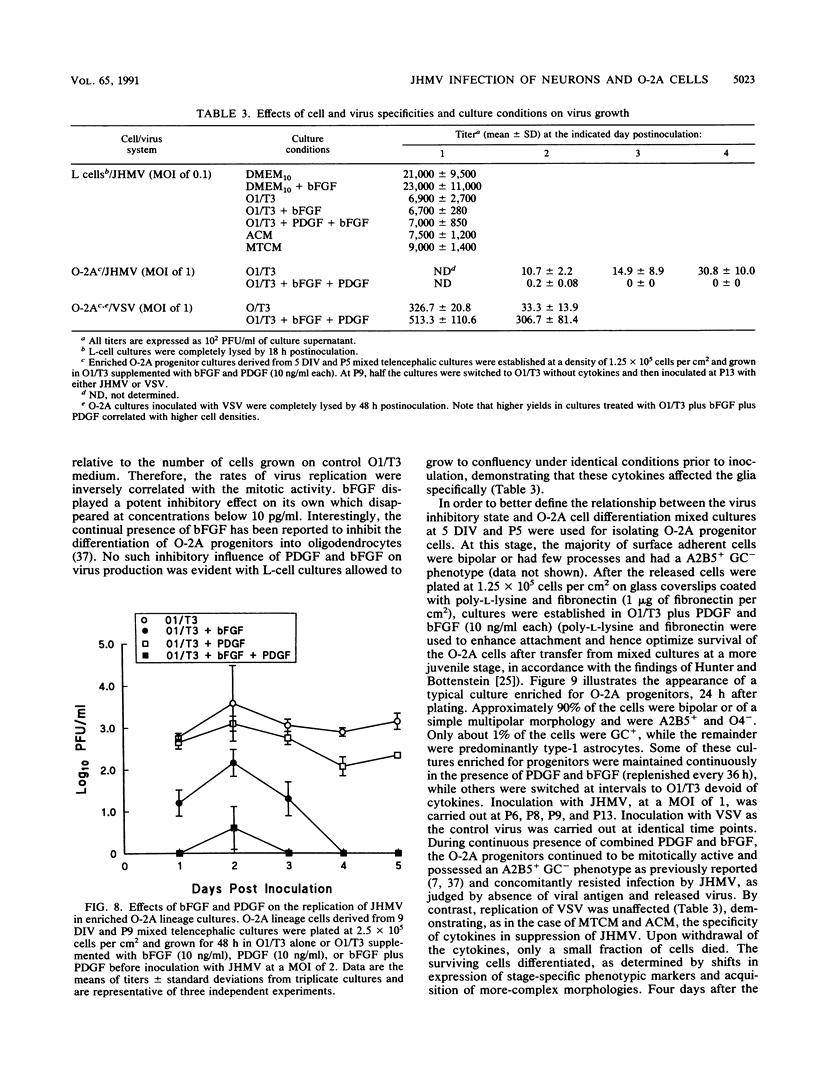
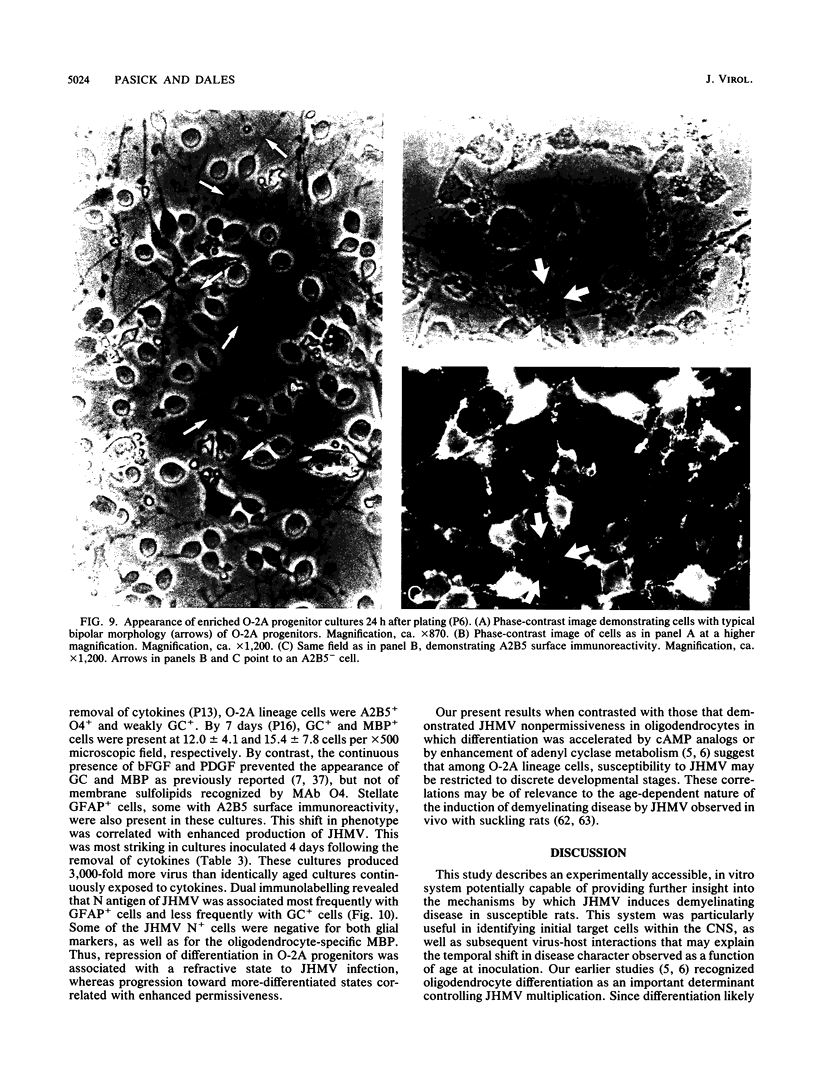
Primary telencephalic cultures derived from neonatal Wistar Furth rats were able to support the growth of coronavirus JHM if a viable neuronal population was maintained. This occurred under serum-free defined, but not serum-supplemented, growth conditions. The importance of neurons in establishing infections in mixed cultures was confirmed by immunocytochemical and electron microscopic studies. Glia, although more abundant than neurons in these cultures, were less frequently infected during the initial 48 h postinoculation. The two glial lineages present in mixed telencephalic cultures were separated into type-1 astrocytes and oligodendrocyte-type-2 astrocyte (O-2A) lineage cells and individually assessed for their ability to support virus growth. Infection could not be established in type-1 astrocytes regardless of the culture conditions employed, consistent with our previous study (S. Beushausen and S. Dales, Virology 141:89-101, 1985). In contrast, infections could be initiated in selected O-2A lineage cells grown in serum-free medium. Virus multiplication was however significantly reduced by preconditioning the medium with mixed telencephalic or enriched type-1 astrocyte cultures, suggesting that intercellular interactions mediated by soluble factor(s) can influence the infectious process in O-2A lineage cells. This presumption was supported by eliciting similar effects with basic fibroblast growth factor and platelet-derived growth factor, two central nervous system cytokines known to control O-2A differentiation. The presence of these cytokines, which synergistically block O-2A cells from differentiating into oligodendrocytes was correlated with specific and reversible resistance to JHM virus (JHMV) infection. These data, combined with our finding that accelerated terminal differentiation of the oligodendrocyte phenotype confers resistance to JHMV (Beushausen and Dales, Virology, 1985), suggest that the permissiveness of O-2A cells for JHMV is restricted to a discrete developmental stage.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizenman Y., de Vellis J. Synergistic action of thyroid hormone, insulin and hydrocortisone on astrocyte differentiation. Brain Res. 1987 Jun 30;414(2):301–308. doi: 10.1016/0006-8993(87)90010-2. [DOI] [PubMed] [Google Scholar]
- Almazan G., Honegger P., Matthieu J. M. Triiodothyronine stimulation of oligodendroglial differentiation and myelination. A developmental study. Dev Neurosci. 1985;7(1):45–54. doi: 10.1159/000112275. [DOI] [PubMed] [Google Scholar]
- Armstrong R. C., Harvath L., Dubois-Dalcq M. E. Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. J Neurosci Res. 1990 Nov;27(3):400–407. doi: 10.1002/jnr.490270319. [DOI] [PubMed] [Google Scholar]
- Behar T., McMorris F. A., Novotný E. A., Barker J. L., Dubois-Dalcq M. Growth and differentiation properties of O-2A progenitors purified from rat cerebral hemispheres. J Neurosci Res. 1988 Oct-Dec;21(2-4):168–180. doi: 10.1002/jnr.490210209. [DOI] [PubMed] [Google Scholar]
- Beushausen S., Dales S. In vivo and in vitro models of demyelinating disease. XI. Tropism and differentiation regulate the infectious process of coronaviruses in primary explants of the rat CNS. Virology. 1985 Feb;141(1):89–101. doi: 10.1016/0042-6822(85)90185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beushausen S., Narindrasorasak S., Sanwal B. D., Dales S. In vivo and in vitro models of demyelinating disease: activation of the adenylate cyclase system influences JHM virus expression in explanted rat oligodendrocytes. J Virol. 1987 Dec;61(12):3795–3803. doi: 10.1128/jvi.61.12.3795-3803.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein J. E. Growth requirements in vitro of oligodendrocyte cell lines and neonatal rat brain oligodendrocytes. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1955–1959. doi: 10.1073/pnas.83.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Dalziel R. G., Koolen M. J. Coronavirus-induced CNS disease: a model for virus-induced demyelination. J Neuroimmunol. 1988 Dec;20(2-3):111–116. doi: 10.1016/0165-5728(88)90141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögler O., Wren D., Barnett S. C., Land H., Noble M. Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6368–6372. doi: 10.1073/pnas.87.16.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R., Cohen J., Fok-Seang J., Hanley M. R., Gregson N. A., Reynolds R., Wilkin G. P. Development of macroglial cells in rat cerebellum. I. Use of antibodies to follow early in vivo development and migration of oligodendrocytes. J Neurocytol. 1988 Feb;17(1):43–54. doi: 10.1007/BF01735376. [DOI] [PubMed] [Google Scholar]
- Dales S., Hanafusa H. Penetration and intracellular release of the genomes of avian RNA tumor viruses. Virology. 1972 Nov;50(2):440–458. doi: 10.1016/0042-6822(72)90396-0. [DOI] [PubMed] [Google Scholar]
- Dalziel R. G., Lampert P. W., Talbot P. J., Buchmeier M. J. Site-specific alteration of murine hepatitis virus type 4 peplomer glycoprotein E2 results in reduced neurovirulence. J Virol. 1986 Aug;59(2):463–471. doi: 10.1128/jvi.59.2.463-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois C., Manuguerra J. C., Hauttecoeur B., Maze J. Monoclonal antibody A2B5, which detects cell surface antigens, binds to ganglioside GT3 (II3 (NeuAc)3LacCer) and to its 9-O-acetylated derivative. J Biol Chem. 1990 Feb 15;265(5):2797–2803. [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P., Lönnerberg P., Ayer-LeLievre C., Persson H. Developmental and regional expression of basic fibroblast growth factor mRNA in the rat central nervous system. J Neurosci Res. 1990 Sep;27(1):10–15. doi: 10.1002/jnr.490270103. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Raff M. C. The oligodendrocyte-type-2 astrocyte cell lineage is specialized for myelination. 1986 Sep 25-Oct 1Nature. 323(6086):335–338. doi: 10.1038/323335a0. [DOI] [PubMed] [Google Scholar]
- Fleming J. O., Trousdale M. D., el-Zaatari F. A., Stohlman S. A., Weiner L. P. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J Virol. 1986 Jun;58(3):869–875. doi: 10.1128/jvi.58.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard A. L., Pfeiffer S. E. Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development. 1989 May;106(1):119–132. doi: 10.1242/dev.106.1.119. [DOI] [PubMed] [Google Scholar]
- Gard A. L., Pfeiffer S. E. Two proliferative stages of the oligodendrocyte lineage (A2B5+O4- and O4+GalC-) under different mitogenic control. Neuron. 1990 Nov;5(5):615–625. doi: 10.1016/0896-6273(90)90216-3. [DOI] [PubMed] [Google Scholar]
- Gavaret J. M., Toru-Delbauffe D., Baghdassarian-Chalaye D., Pomerance M., Pierre M. Thyroid hormone action: induction of morphological changes and protein secretion in astroglial cell cultures. Brain Res Dev Brain Res. 1991 Jan 15;58(1):43–49. doi: 10.1016/0165-3806(91)90235-b. [DOI] [PubMed] [Google Scholar]
- Gilad G. M., Shanker G., Dahl D., Gilad V. H. Dibutyryl cyclic AMP-induced changes in neuron-astroglia interactions and fibronectin immunocytochemistry in dissociated rat cerebellar cultures. Brain Res. 1990 Feb 5;508(2):215–224. doi: 10.1016/0006-8993(90)90399-v. [DOI] [PubMed] [Google Scholar]
- Godfraind C., Friedrich V. L., Holmes K. V., Dubois-Dalcq M. In vivo analysis of glial cell phenotypes during a viral demyelinating disease in mice. J Cell Biol. 1989 Nov;109(5):2405–2416. doi: 10.1083/jcb.109.5.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten M. E., Lynch M., Rydel R. E., Sanchez J., Joseph-Silverstein J., Moscatelli D., Rifkin D. B. In vitro neurite extension by granule neurons is dependent upon astroglial-derived fibroblast growth factor. Dev Biol. 1988 Feb;125(2):280–289. doi: 10.1016/0012-1606(88)90211-4. [DOI] [PubMed] [Google Scholar]
- Hunter S. F., Bottenstein J. E. Bipotential glial progenitors are targets of neuronal cell line-derived growth factors. Brain Res Dev Brain Res. 1989 Sep 1;49(1):33–49. doi: 10.1016/0165-3806(89)90057-6. [DOI] [PubMed] [Google Scholar]
- Hunter S. F., Bottenstein J. E. Growth factor responses of enriched bipotential glial progenitors. Brain Res Dev Brain Res. 1990 Jul 1;54(2):235–248. doi: 10.1016/0165-3806(90)90146-p. [DOI] [PubMed] [Google Scholar]
- Ingraham C. A., McCarthy K. D. Plasticity of process-bearing glial cell cultures from neonatal rat cerebral cortical tissue. J Neurosci. 1989 Jan;9(1):63–69. doi: 10.1523/JNEUROSCI.09-01-00063.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. U. Neurobiology of human oligodendrocytes in culture. J Neurosci Res. 1990 Dec;27(4):712–728. doi: 10.1002/jnr.490270432. [DOI] [PubMed] [Google Scholar]
- Levine J. M. Neuronal influences on glial progenitor cell development. Neuron. 1989 Jul;3(1):103–113. doi: 10.1016/0896-6273(89)90119-0. [DOI] [PubMed] [Google Scholar]
- Lillien L. E., Raff M. C. Analysis of the cell-cell interactions that control type-2 astrocyte development in vitro. Neuron. 1990 Apr;4(4):525–534. doi: 10.1016/0896-6273(90)90110-2. [DOI] [PubMed] [Google Scholar]
- Lillien L. E., Raff M. C. Differentiation signals in the CNS: type-2 astrocyte development in vitro as a model system. Neuron. 1990 Aug;5(2):111–119. doi: 10.1016/0896-6273(90)90301-u. [DOI] [PubMed] [Google Scholar]
- Lillien L. E., Sendtner M., Raff M. C. Extracellular matrix-associated molecules collaborate with ciliary neurotrophic factor to induce type-2 astrocyte development. J Cell Biol. 1990 Aug;111(2):635–644. doi: 10.1083/jcb.111.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A., Coulter M., Anderson R., Dales S., Flintoff W. In vivo and in vitro models of demyelinating diseases. II. Persistence and host-regulated thermosensitivity in cells of neural derivation infected with mouse hepatitis and measles viruses. Virology. 1978 Jul 15;88(2):325–337. doi: 10.1016/0042-6822(78)90289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa P. T., Wege H., ter Meulen V. Analysis of murine hepatitis virus (JHM strain) tropism toward Lewis rat glial cells in vitro. Type I astrocytes and brain macrophages (microglia) as primary glial cell targets. Lab Invest. 1986 Sep;55(3):318–327. [PubMed] [Google Scholar]
- Massa P. T., Wege H., ter Meulen V. Growth pattern of various JHM coronavirus isolates in primary rat glial cell cultures correlates with differing neurotropism in vivo. Virus Res. 1988 Feb;9(2-3):133–144. doi: 10.1016/0168-1702(88)90028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massalski A., Coulter-Mackie M., Knobler R. L., Buchmeier M. J., Dales S. In vivo and in vitro models of demyelinating diseases. V. Comparison of the assembly of mouse hepatitis virus, strain JHM, in two murine cell lines. Intervirology. 1982;18(3):135–146. doi: 10.1159/000149316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon R. D., Matsui T., Dubois-Dalcq M., Aaronson S. A. FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron. 1990 Nov;5(5):603–614. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- McMorris F. A. Cyclic AMP induction of the myelin enzyme 2',3'-cyclic nucleotide 3'-phosphohydrolase in rat oligodendrocytes. J Neurochem. 1983 Aug;41(2):506–515. doi: 10.1111/j.1471-4159.1983.tb04768.x. [DOI] [PubMed] [Google Scholar]
- Morris V. L., Tieszer C., Mackinnon J., Percy D. Characterization of coronavirus JHM variants isolated from Wistar Furth rats with a viral-induced demyelinating disease. Virology. 1989 Mar;169(1):127–136. doi: 10.1016/0042-6822(89)90048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka S., Takahashi T. Primary dissociated cell culture of fetal rat central nervous tissue. II. Immunocytochemical and ultrastructural studies of myelinogenesis. Brain Res Dev Brain Res. 1989 Sep 1;49(1):63–74. doi: 10.1016/0165-3806(89)90059-x. [DOI] [PubMed] [Google Scholar]
- Nagashima K., Wege H., Meyermann R., ter Meulen V. Corona virus induced subacute demyelinating encephalomyelitis in rats: a morphological analysis. Acta Neuropathol. 1978 Oct 13;44(1):63–70. doi: 10.1007/BF00691641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K., Wege H., Meyermann R., ter Meulen V. Demyelinating encephalomyelitis induced by a long-term corona virus infection in rats. A preliminary report. Acta Neuropathol. 1979 Mar 15;45(3):205–213. doi: 10.1007/BF00702672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M., Murray K., Stroobant P., Waterfield M. D., Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988 Jun 9;333(6173):560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Nunez J. Effects of thyroid hormones during brain differentiation. Mol Cell Endocrinol. 1984 Sep;37(2):125–132. doi: 10.1016/0303-7207(84)90043-1. [DOI] [PubMed] [Google Scholar]
- Parham D., Tereba A., Talbot P. J., Jackson D. P., Morris V. L. Analysis of JHM central nervous system infections in rats. Arch Neurol. 1986 Jul;43(7):702–708. doi: 10.1001/archneur.1986.00520070058019. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Cohen J., Lindsay R., Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983 Jun;3(6):1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Miller R. H. Two glial cell lineages diverge prenatally in rat optic nerve. Dev Biol. 1984 Nov;106(1):53–60. doi: 10.1016/0012-1606(84)90060-5. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Glial cell diversification in the rat optic nerve. Science. 1989 Mar 17;243(4897):1450–1455. doi: 10.1126/science.2648568. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Lillien L. E., Richardson W. D., Burne J. F., Noble M. D. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988 Jun 9;333(6173):562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Raible D. W., McMorris F. A. Cyclic AMP regulates the rate of differentiation of oligodendrocytes without changing the lineage commitment of their progenitors. Dev Biol. 1989 Jun;133(2):437–446. doi: 10.1016/0012-1606(89)90047-x. [DOI] [PubMed] [Google Scholar]
- Raible D. W., McMorris F. A. Induction of oligodendrocyte differentiation by activators of adenylate cyclase. J Neurosci Res. 1990 Sep;27(1):43–46. doi: 10.1002/jnr.490270107. [DOI] [PubMed] [Google Scholar]
- Rauvala H. An 18-kd heparin-binding protein of developing brain that is distinct from fibroblast growth factors. EMBO J. 1989 Oct;8(10):2933–2941. doi: 10.1002/j.1460-2075.1989.tb08443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Pringle N., Mosley M. J., Westermark B., Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988 Apr 22;53(2):309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Sasahara M., Fries J. W., Raines E. W., Gown A. M., Westrum L. E., Frosch M. P., Bonthron D. T., Ross R., Collins T. PDGF B-chain in neurons of the central nervous system, posterior pituitary, and in a transgenic model. Cell. 1991 Jan 11;64(1):217–227. doi: 10.1016/0092-8674(91)90223-l. [DOI] [PubMed] [Google Scholar]
- Sato Y., Murphy P. R., Sato R., Friesen H. G. Fibroblast growth factor release by bovine endothelial cells and human astrocytoma cells in culture is density dependent. Mol Endocrinol. 1989 Apr;3(4):744–748. doi: 10.1210/mend-3-4-744. [DOI] [PubMed] [Google Scholar]
- Sommer I., Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981 Apr 30;83(2):311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Sorensen O., Coulter-Mackie M. B., Puchalski S., Dales S. In vivo and in vitro models of demyelinating disease. IX. Progression of JHM virus infection in the central nervous system of the rat during overt and asymptomatic phases. Virology. 1984 Sep;137(2):347–357. doi: 10.1016/0042-6822(84)90227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen O., Dales S. In vivo and in vitro models of demyelinating disease: JHM virus in the rat central nervous system localized by in situ cDNA hybridization and immunofluorescent microscopy. J Virol. 1985 Nov;56(2):434–438. doi: 10.1128/jvi.56.2.434-438.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen O., Dugre R., Percy D., Dales S. In vivo and in vitro models of demyelinating disease: endogenous factors influencing demyelinating disease caused by mouse hepatitis virus in rats and mice. Infect Immun. 1982 Sep;37(3):1248–1260. doi: 10.1128/iai.37.3.1248-1260.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen O., Perry D., Dales S. In vivo and in vitro models of demyelinating diseases. III. JHM virus infection of rats. Arch Neurol. 1980 Aug;37(8):478–484. doi: 10.1001/archneur.1980.00500570026003. [DOI] [PubMed] [Google Scholar]
- Sorensen O., Saravani A., Dales S. In vivo and in vitro models of demyelinating disease. XVII. The infectious process in athymic rats inoculated with JHM virus. Microb Pathog. 1987 Feb;2(2):79–90. doi: 10.1016/0882-4010(87)90100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P. J., Buchmeier M. J. Antigenic variation among murine coronaviruses: evidence for polymorphism on the peplomer glycoprotein, E2. Virus Res. 1985 Jun;2(4):317–328. doi: 10.1016/0168-1702(85)90028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torelli S., Dell'Era P., Ennas M. G., Sogos V., Gremo F., Ragnotti G., Presta M. Basic fibroblast growth factor in neuronal cultures of human fetal brain. J Neurosci Res. 1990 Sep;27(1):78–83. doi: 10.1002/jnr.490270112. [DOI] [PubMed] [Google Scholar]
- Vaysse P. J., Goldman J. E. A clonal analysis of glial lineages in neonatal forebrain development in vitro. Neuron. 1990 Sep;5(3):227–235. doi: 10.1016/0896-6273(90)90160-h. [DOI] [PubMed] [Google Scholar]
- Wege H., Winter J., Meyermann R. The peplomer protein E2 of coronavirus JHM as a determinant of neurovirulence: definition of critical epitopes by variant analysis. J Gen Virol. 1988 Jan;69(Pt 1):87–98. doi: 10.1099/0022-1317-69-1-87. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Beushausen S., Dales S. In vivo and in vitro models of demyelinating diseases. XV. Differentiation influences the regulation of coronavirus infection in primary explants of mouse CNS. Virology. 1986 Jun;151(2):253–264. doi: 10.1016/0042-6822(86)90047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H. J., Ruit K. G., Wang Y. X., Parks W. C., Snider W. D., Deuel T. F. PDGF A-chain gene is expressed by mammalian neurons during development and in maturity. Cell. 1991 Jan 11;64(1):209–216. doi: 10.1016/0092-8674(91)90222-k. [DOI] [PubMed] [Google Scholar]
- Zimmer M. J., Dales S. In vivo and in vitro models of demyelinating diseases. XXIV. The infectious process in cyclosporin A treated Wistar Lewis rats inoculated with JHM virus. Microb Pathog. 1989 Jan;6(1):7–16. doi: 10.1016/0882-4010(89)90003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berlo M. F., Warringa R., Wolswijk G., Lopes-Cardozo M. Vulnerability of rat and mouse brain cells to murine hepatitis virus (JHM-strain): studies in vivo and in vitro. Glia. 1989;2(2):85–93. doi: 10.1002/glia.440020204. [DOI] [PMC free article] [PubMed] [Google Scholar]