Abstract
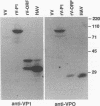
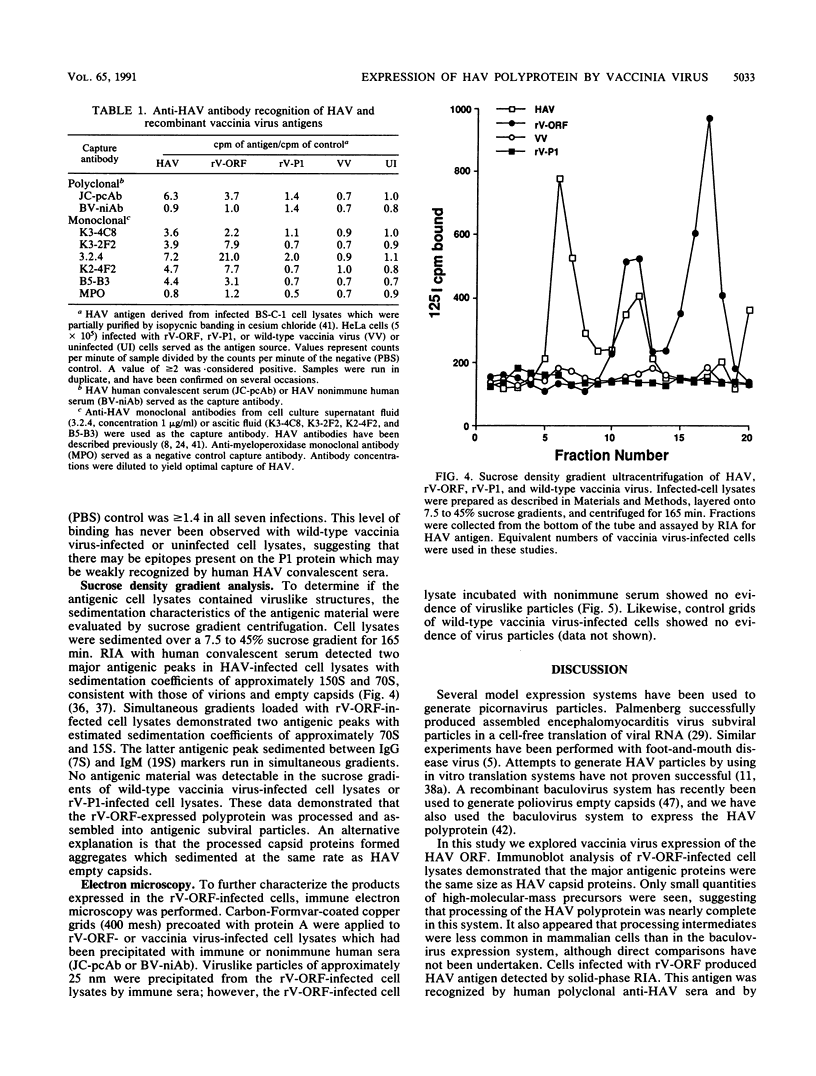
Hepatitis A virus (HAV) contains a single-stranded, plus-sense RNA genome with a single long open reading frame encoding a polyprotein of approximately 250 kDa. Viral structural proteins are generated by posttranslational proteolytic processing of this polyprotein. We constructed recombinant vaccinia viruses which expressed the HAV polyprotein (rV-ORF) and the P1 structural region (rV-P1). rV-ORF-infected cell lysates demonstrated that the polyprotein was cleaved into immunoreactive 29- and 33-kDa proteins which comigrated with HAV capsid proteins VP0 and VP1. The rV-P1 construct produced a 90-kDa protein which showed no evidence of posttranslational processing. Solid-phase radioimmunoassays with human polyclonal anti-HAV sera and with murine or human neutralizing monoclonal anti-HAV antibodies recognized the rV-ORF-infected cell lysates. Sucrose density gradients of rV-ORF-infected cell lysates contained peaks of HAV antigen with sedimentation coefficients of approximately 70S and 15S, similar to those of HAV empty capsids and pentamers. Immune electron microscopy also demonstrated the presence of viruslike particles in rV-ORF-infected cell lysates. Thus, the HAV polyprotein expressed by a recombinant vaccinia virus demonstrated posttranslational processing into mature capsid proteins which assembled into antigenic viruslike particles.
Full text
PDF

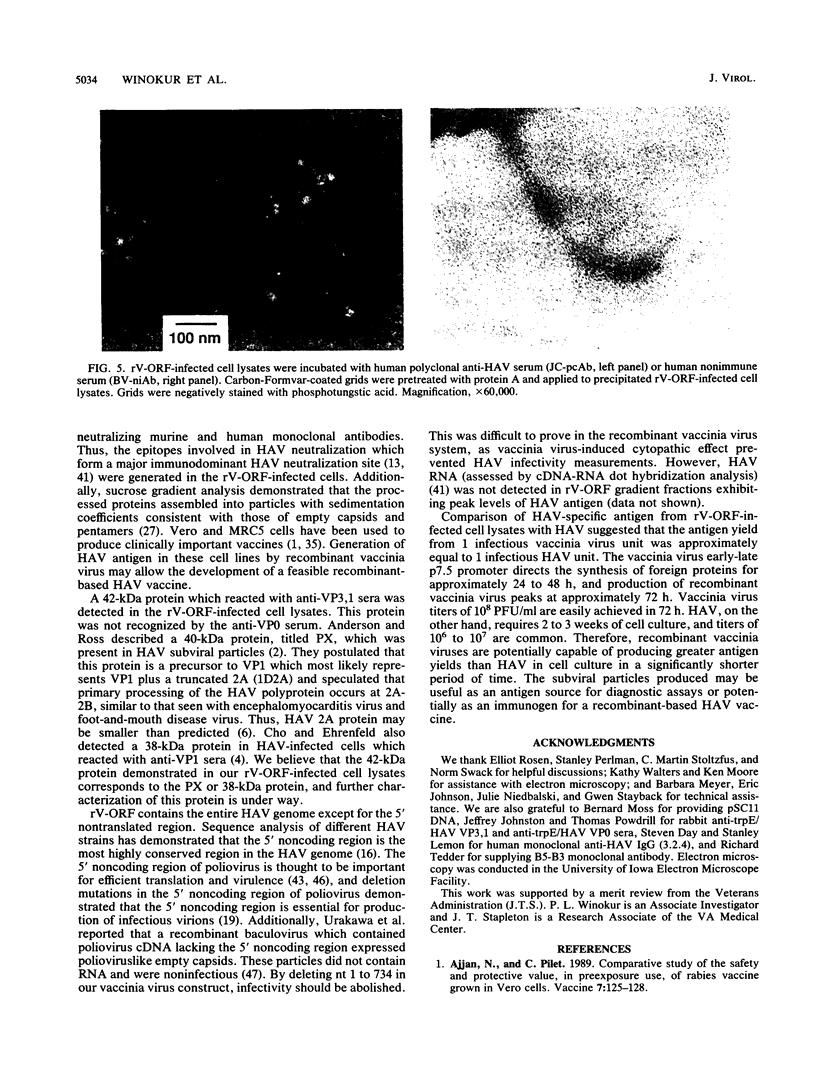


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajjan N., Pilet C. Comparative study of the safety and protective value, in pre-exposure use, of rabies vaccine cultivated on human diploid cells (HDCV) and of the new vaccine grown on Vero cells. Vaccine. 1989 Apr;7(2):125–128. doi: 10.1016/0264-410x(89)90050-9. [DOI] [PubMed] [Google Scholar]
- Anderson D. A., Ross B. C. Morphogenesis of hepatitis A virus: isolation and characterization of subviral particles. J Virol. 1990 Nov;64(11):5284–5289. doi: 10.1128/jvi.64.11.5284-5289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M. W., Ehrenfeld E. Rapid completion of the replication cycle of hepatitis A virus subsequent to reversal of guanidine inhibition. Virology. 1991 Feb;180(2):770–780. doi: 10.1016/0042-6822(91)90090-x. [DOI] [PubMed] [Google Scholar]
- Clarke B. E., Sangar D. V. Processing and assembly of foot-and-mouth disease virus proteins using subgenomic RNA. J Gen Virol. 1988 Sep;69(Pt 9):2313–2325. doi: 10.1099/0022-1317-69-9-2313. [DOI] [PubMed] [Google Scholar]
- Cohen J. I., Ticehurst J. R., Purcell R. H., Buckler-White A., Baroudy B. M. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987 Jan;61(1):50–59. doi: 10.1128/jvi.61.1.50-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Hughes J. V., Perlow D. S., Boger J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol. 1985 Sep;55(3):836–839. doi: 10.1128/jvi.55.3.836-839.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flehmig B., Heinricy U., Pfisterer M. Immunogenicity of a killed hepatitis A vaccine in seronegative volunteers. Lancet. 1989 May 13;1(8646):1039–1041. doi: 10.1016/s0140-6736(89)92443-4. [DOI] [PubMed] [Google Scholar]
- Gauss-Müller V., von der Helm K., Deinhardt F. Translation in vitro of hepatitis A virus RNA. Virology. 1984 Aug;137(1):182–184. doi: 10.1016/0042-6822(84)90021-7. [DOI] [PubMed] [Google Scholar]
- Hughes J. V., Stanton L. W., Tomassini J. E., Long W. J., Scolnick E. M. Neutralizing monoclonal antibodies to hepatitis A virus: partial localization of a neutralizing antigenic site. J Virol. 1984 Nov;52(2):465–473. doi: 10.1128/jvi.52.2.465-473.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey C. D., Cook E. H., Jr, Bradley D. W. Identification of enterically transmitted hepatitis virus particles by solid phase immune electron microscopy. J Virol Methods. 1990 Aug;29(2):177–188. doi: 10.1016/0166-0934(90)90111-r. [DOI] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Greengard P. A quantitative dot-immunobinding assay for proteins using nitrocellulose membrane filters. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1684–1687. doi: 10.1073/pnas.81.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R. W., Newbold J. E., Lemon S. M. Complete nucleotide sequence of a cell culture-adapted variant of hepatitis A virus: comparison with wild-type virus with restricted capacity for in vitro replication. Virology. 1988 Apr;163(2):299–307. doi: 10.1016/0042-6822(88)90270-x. [DOI] [PubMed] [Google Scholar]
- Johnston J. M., Harmon S. A., Binn L. N., Richards O. C., Ehrenfeld E., Summers D. F. Antigenic and immunogenic properties of a hepatitis A virus capsid protein expressed in Escherichia coli. J Infect Dis. 1988 Jun;157(6):1203–1211. doi: 10.1093/infdis/157.6.1203. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kuge S., Nomoto A. Construction of viable deletion and insertion mutants of the Sabin strain of type 1 poliovirus: function of the 5' noncoding sequence in viral replication. J Virol. 1987 May;61(5):1478–1487. doi: 10.1128/jvi.61.5.1478-1487.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K., Veit M., Klenk H. D. Retarded processing of influenza virus hemagglutinin in insect cells. Virology. 1991 Jan;180(1):159–165. doi: 10.1016/0042-6822(91)90019-8. [DOI] [PubMed] [Google Scholar]
- Lemon S. M., Binn L. N., Marchwicki R. H. Radioimmunofocus assay for quantitation of hepatitis A virus in cell cultures. J Clin Microbiol. 1983 May;17(5):834–839. doi: 10.1128/jcm.17.5.834-839.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M., Jansen R. W., Newbold J. E. Infectious hepatitis A virus particles produced in cell culture consist of three distinct types with different buoyant densities in CsCl. J Virol. 1985 Apr;54(1):78–85. doi: 10.1128/jvi.54.1.78-85.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor A., Kornitschuk M., Hurrell J. G., Lehmann N. I., Coulepis A. G., Locarnini S. A., Gust I. D. Monoclonal antibodies against hepatitis A virus. J Clin Microbiol. 1983 Nov;18(5):1237–1243. doi: 10.1128/jcm.18.5.1237-1243.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J. S., Dong D. X., Zhang H. Y., Chen N. L., Zhang X. Y., Huang H. Y., Xie R. Y., Zhou T. J., Wan Z. J., Wang Y. Z. Primary study of attenuated live hepatitis A vaccine (H2 strain) in humans. J Infect Dis. 1989 Apr;159(4):621–624. doi: 10.1093/infdis/159.4.621. [DOI] [PubMed] [Google Scholar]
- Palmenberg A. C. In vitro synthesis and assembly of picornaviral capsid intermediate structures. J Virol. 1982 Dec;44(3):900–906. doi: 10.1128/jvi.44.3.900-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia A. T., Nielsen L., Armington L., Thurman D. J., Tierney E., Nichols C. R. A community-wide outbreak of hepatitis A in a religious community: impact of mass administration of immune globulin. Am J Epidemiol. 1990 Jun;131(6):1085–1093. doi: 10.1093/oxfordjournals.aje.a115601. [DOI] [PubMed] [Google Scholar]
- Ping L. H., Jansen R. W., Stapleton J. T., Cohen J. I., Lemon S. M. Identification of an immunodominant antigenic site involving the capsid protein VP3 of hepatitis A virus. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8281–8285. doi: 10.1073/pnas.85.21.8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powdrill T. F., Johnston J. M. Immunologic priming with recombinant hepatitis A virus capsid proteins produced in Escherichia coli. J Virol. 1991 May;65(5):2686–2690. doi: 10.1128/jvi.65.5.2686-2690.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Picornaviral structure and assembly. Microbiol Rev. 1981 Jun;45(2):287–315. doi: 10.1128/mr.45.2.287-315.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd W. M., Langford D. T., Kelly A. A single-blind, placebo-controlled comparison of the reactivity and antigenicity of trivalent oral poliomyelitis vaccine prepared in monkey kidney or human diploid cell substrates. J Biol Stand. 1983 Jan;11(1):29–33. doi: 10.1016/s0092-1157(83)80043-2. [DOI] [PubMed] [Google Scholar]
- Siegl G., Frösner G. G. Characterization and classification of virus particles associated with hepatitis A. I. Size, density, and sedimentation. J Virol. 1978 Apr;26(1):40–47. doi: 10.1128/jvi.26.1.40-47.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Frösner G. G., Gauss-Müller V., Tratschin J. D., Deinhardt F. The physicochemical properties of infectious hepatitis A virions. J Gen Virol. 1981 Dec;57(Pt 2):331–341. doi: 10.1099/0022-1317-57-2-331. [DOI] [PubMed] [Google Scholar]
- Sjogren M. H., Hoke C. H., Binn L. N., Eckels K. H., Dubois D. R., Lyde L., Tsuchida A., Oaks S., Jr, Marchwicki R., Lednar W. Immunogenicity of an inactivated hepatitis A vaccine. Ann Intern Med. 1991 Mar 15;114(6):470–471. doi: 10.7326/0003-4819-114-6-470. [DOI] [PubMed] [Google Scholar]
- Stapleton J. T., Jansen R., Lemon S. M. Neutralizing antibody to hepatitis A virus in immune serum globulin and in the sera of human recipients of immune serum globulin. Gastroenterology. 1985 Sep;89(3):637–642. doi: 10.1016/0016-5085(85)90462-7. [DOI] [PubMed] [Google Scholar]
- Stapleton J. T., Lange D. K., LeDuc J. W., Binn L. N., Jansen R. W., Lemon S. M. The role of secretory immunity in hepatitis A virus infection. J Infect Dis. 1991 Jan;163(1):7–11. doi: 10.1093/infdis/163.1.7. [DOI] [PubMed] [Google Scholar]
- Stapleton J. T., Lemon S. M. Neutralization escape mutants define a dominant immunogenic neutralization site on hepatitis A virus. J Virol. 1987 Feb;61(2):491–498. doi: 10.1128/jvi.61.2.491-498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin G. J., Young D. C., Flanegan J. B. Self-catalyzed linkage of poliovirus terminal protein VPg to poliovirus RNA. Cell. 1989 Nov 3;59(3):511–519. doi: 10.1016/0092-8674(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H., Nicklin M. J., Murray M. G., Anderson C. W., Dunn J. J., Studier F. W., Wimmer E. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell. 1986 Jun 6;45(5):761–770. doi: 10.1016/0092-8674(86)90790-7. [DOI] [PubMed] [Google Scholar]
- Trono D., Andino R., Baltimore D. An RNA sequence of hundreds of nucleotides at the 5' end of poliovirus RNA is involved in allowing viral protein synthesis. J Virol. 1988 Jul;62(7):2291–2299. doi: 10.1128/jvi.62.7.2291-2299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa T., Ferguson M., Minor P. D., Cooper J., Sullivan M., Almond J. W., Bishop D. H. Synthesis of immunogenic, but non-infectious, poliovirus particles in insect cells by a baculovirus expression vector. J Gen Virol. 1989 Jun;70(Pt 6):1453–1463. doi: 10.1099/0022-1317-70-6-1453. [DOI] [PubMed] [Google Scholar]
- Wiedermann G., Ambrosch F., Kollaritsch H., Hofmann H., Kunz C., D'Hondt E., Delem A., André F. E., Safary A., Stéphenne J. Safety and immunogenicity of an inactivated hepatitis A candidate vaccine in healthy adult volunteers. Vaccine. 1990 Dec;8(6):581–584. doi: 10.1016/0264-410x(90)90013-c. [DOI] [PubMed] [Google Scholar]