Abstract
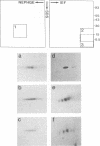
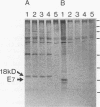
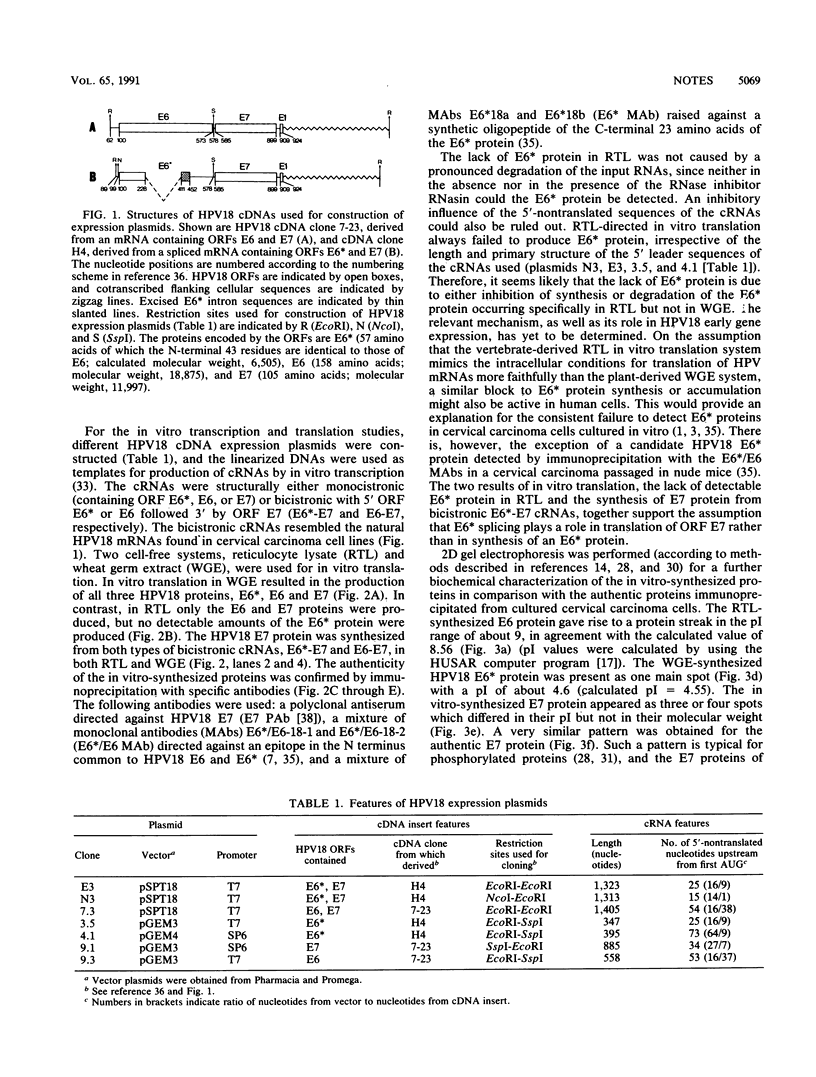
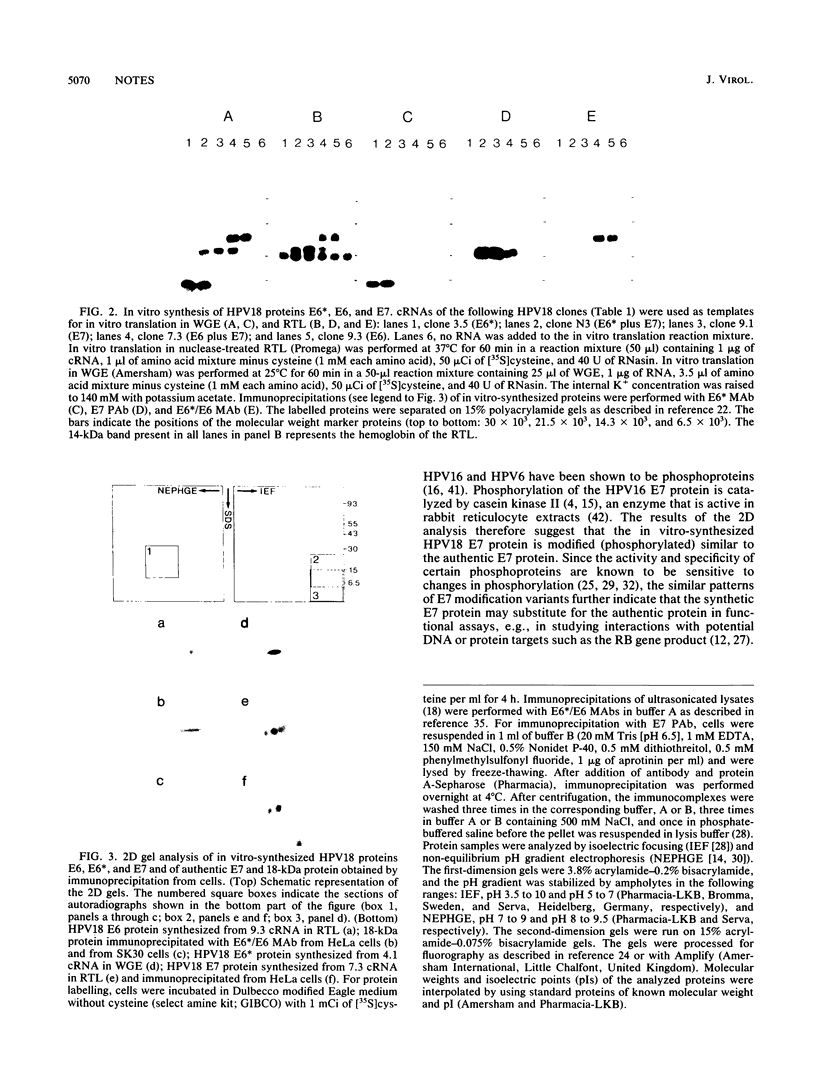
Expression of the E6 and E7 transforming genes of human papillomavirus type 18 (HPV18) occurs via structurally bicistronic mRNAs in which the downstream open reading frame (ORF) E7 is preceded either by the full-length ORF E6 or by a spliced ORF, E6*. We have used in vitro transcription and translation of HPV18 cDNAs in order to analyze the synthesis of E6*, E6, and E7 proteins and to compare the E6 and E7 in vitro translation products with the authentic proteins immunoprecipitated from cervical cancer cells. In wheat germ extract, in vitro translation resulted in the production of all three proteins, E6*, E6, and E7. In rabbit reticulocyte lysate, however, only the E6 and E7 proteins were produced. The lack of E6* protein was due neither to template RNA degradation nor to an inhibitory influence of the RNA 5' leader sequences, thus indicating the possibility of either inhibition of synthesis or degradation of E6* protein in reticulocyte lysate. The E7 protein was synthesized from both E6*-E7 and E6-E7 RNAs. In vitro-synthesized and authentic HPV18 E7 proteins revealed identical electrophoretic mobilities in two-dimensional gel electrophoresis, thus indicating similar modifications. By using a monoclonal antibody against the N terminus of HPV18 E6* and E6, an 18-kDa protein was detected not only in HPV18-positive but also in HPV18-negative epithelial cells. The 18-kDa proteins and the in vitro-synthesized HPV18 E6 protein exhibited comparable electrophoretic characteristics in two-dimensional gels. These results suggest the possible existence of a cellular protein related to HPV18 E6.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Androphy E. J., Hubbert N. L., Schiller J. T., Lowy D. R. Identification of the HPV-16 E6 protein from transformed mouse cells and human cervical carcinoma cell lines. EMBO J. 1987 Apr;6(4):989–992. doi: 10.1002/j.1460-2075.1987.tb04849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. C., Phelps W. C., Lindgren V., Braun M. J., Gonda M. A., Howley P. M. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J Virol. 1987 Apr;61(4):962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks L., Spence P., Androphy E., Hubbert N., Matlashewski G., Murray A., Crawford L. Identification of human papillomavirus type 18 E6 polypeptide in cells derived from human cervical carcinomas. J Gen Virol. 1987 May;68(Pt 5):1351–1359. doi: 10.1099/0022-1317-68-5-1351. [DOI] [PubMed] [Google Scholar]
- Barbosa M. S., Edmonds C., Fisher C., Schiller J. T., Lowy D. R., Vousden K. H. The region of the HPV E7 oncoprotein homologous to adenovirus E1a and Sv40 large T antigen contains separate domains for Rb binding and casein kinase II phosphorylation. EMBO J. 1990 Jan;9(1):153–160. doi: 10.1002/j.1460-2075.1990.tb08091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa M. S., Schlegel R. The E6 and E7 genes of HPV-18 are sufficient for inducing two-stage in vitro transformation of human keratinocytes. Oncogene. 1989 Dec;4(12):1529–1532. [PubMed] [Google Scholar]
- Bedell M. A., Jones K. H., Grossman S. R., Laimins L. A. Identification of human papillomavirus type 18 transforming genes in immortalized and primary cells. J Virol. 1989 Mar;63(3):1247–1255. doi: 10.1128/jvi.63.3.1247-1255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleul C., Müller M., Frank R., Gausepohl H., Koldovsky U., Mgaya H. N., Luande J., Pawlita M., ter Meulen J., Viscidi R. Human papillomavirus type 18 E6 and E7 antibodies in human sera: increased anti-E7 prevalence in cervical cancer patients. J Clin Microbiol. 1991 Aug;29(8):1579–1588. doi: 10.1128/jcm.29.8.1579-1588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988 Mar;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Nasseri M., Wolinsky S. M., Broker T. R. Human papillomavirus types 6 and 11 mRNAs from genital condylomata acuminata. J Virol. 1987 Aug;61(8):2581–2588. doi: 10.1128/jvi.61.8.2581-2588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook T., Morgenstern J. P., Crawford L., Banks L. Continued expression of HPV-16 E7 protein is required for maintenance of the transformed phenotype of cells co-transformed by HPV-16 plus EJ-ras. EMBO J. 1989 Feb;8(2):513–519. doi: 10.1002/j.1460-2075.1989.tb03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Firzlaff J. M., Galloway D. A., Eisenman R. N., Lüscher B. The E7 protein of human papillomavirus type 16 is phosphorylated by casein kinase II. New Biol. 1989 Oct;1(1):44–53. [PubMed] [Google Scholar]
- Gage J. R., Meyers C., Wettstein F. O. The E7 proteins of the nononcogenic human papillomavirus type 6b (HPV-6b) and of the oncogenic HPV-16 differ in retinoblastoma protein binding and other properties. J Virol. 1990 Feb;64(2):723–730. doi: 10.1128/jvi.64.2.723-730.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley-Nelson P., Vousden K. H., Hubbert N. L., Lowy D. R., Schiller J. T. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989 Dec 1;8(12):3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J. B., Bedell M. A., McCance D. J., Laiminis L. A. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol. 1990 Feb;64(2):519–526. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo R. D., Lane D. P. Nuclear protein p68 is an RNA-dependent ATPase. EMBO J. 1989 Jun;8(6):1827–1831. doi: 10.1002/j.1460-2075.1989.tb03577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Hoeffler W. K. SV40 large T shares an antigenic determinant with a cellular protein of molecular weight 68,000. Nature. 1980 Nov 13;288(5787):167–170. doi: 10.1038/288167a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lewin B. Driving the cell cycle: M phase kinase, its partners, and substrates. Cell. 1990 Jun 1;61(5):743–752. doi: 10.1016/0092-8674(90)90181-d. [DOI] [PubMed] [Google Scholar]
- Münger K., Phelps W. C., Bubb V., Howley P. M., Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989 Oct;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Werness B. A., Dyson N., Phelps W. C., Harlow E., Howley P. M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989 Dec 20;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H., Edgar B. A., Lakich D., Lehner C. F. Directing cell division during development. Science. 1989 Nov 3;246(4930):635–640. doi: 10.1126/science.2683080. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Peterson J. L., McConkey E. H. Non-histone chromosomal proteins from HeLa cells. A survey by high resolution, two-dimensional electrophoresis. J Biol Chem. 1976 Jan 25;251(2):548–554. [PubMed] [Google Scholar]
- Prives C. The replication functions of SV40 T antigen are regulated by phosphorylation. Cell. 1990 Jun 1;61(5):735–738. doi: 10.1016/0092-8674(90)90179-i. [DOI] [PubMed] [Google Scholar]
- Rotenberg M. O., Chow L. T., Broker T. R. Characterization of rare human papillomavirus type 11 mRNAs coding for regulatory and structural proteins, using the polymerase chain reaction. Virology. 1989 Oct;172(2):489–497. doi: 10.1016/0042-6822(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Kaul S., Schwarz E., Gausepohl H., Frank R., Bastert G. Identification of the human papillomavirus type 18 E6 and E6 proteins in nuclear protein fractions from human cervical carcinoma cells grown in the nude mouse or in vitro. Cancer Res. 1988 Jun 1;48(11):2969–2974. [PubMed] [Google Scholar]
- Schneider-Gädicke A., Schwarz E. Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J. 1986 Sep;5(9):2285–2292. doi: 10.1002/j.1460-2075.1986.tb04496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Oltersdorf T., Krämmer G., Röwekamp W. Identification of early proteins of the human papilloma viruses type 16 (HPV 16) and type 18 (HPV 18) in cervical carcinoma cells. EMBO J. 1987 Jan;6(1):139–144. doi: 10.1002/j.1460-2075.1987.tb04731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Prokoph H., Wettstein F. O. Oncogenic and nononcogenic human genital papillomaviruses generate the E7 mRNA by different mechanisms. J Virol. 1989 Mar;63(3):1441–1447. doi: 10.1128/jvi.63.3.1441-1447.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. The major human papillomavirus protein in cervical cancers is a cytoplasmic phosphoprotein. J Virol. 1987 May;61(5):1686–1689. doi: 10.1128/jvi.61.5.1686-1689.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traugh J. A., Traut R. R. Characterization of protein kinases from rabbit reticulocytes. J Biol Chem. 1974 Feb 25;249(4):1207–1212. [PubMed] [Google Scholar]
- Vousden K. H. Human papillomaviruses and cervical carcinoma. Cancer Cells. 1989 Oct;1(2):43–50. [PubMed] [Google Scholar]
- Werness B. A., Levine A. J., Howley P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- el Awady M. K., Kaplan J. B., O'Brien S. J., Burk R. D. Molecular analysis of integrated human papillomavirus 16 sequences in the cervical cancer cell line SiHa. Virology. 1987 Aug;159(2):389–398. doi: 10.1016/0042-6822(87)90478-8. [DOI] [PubMed] [Google Scholar]
- von Knebel Doeberitz M., Oltersdorf T., Schwarz E., Gissmann L. Correlation of modified human papilloma virus early gene expression with altered growth properties in C4-1 cervical carcinoma cells. Cancer Res. 1988 Jul 1;48(13):3780–3786. [PubMed] [Google Scholar]