Abstract
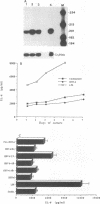
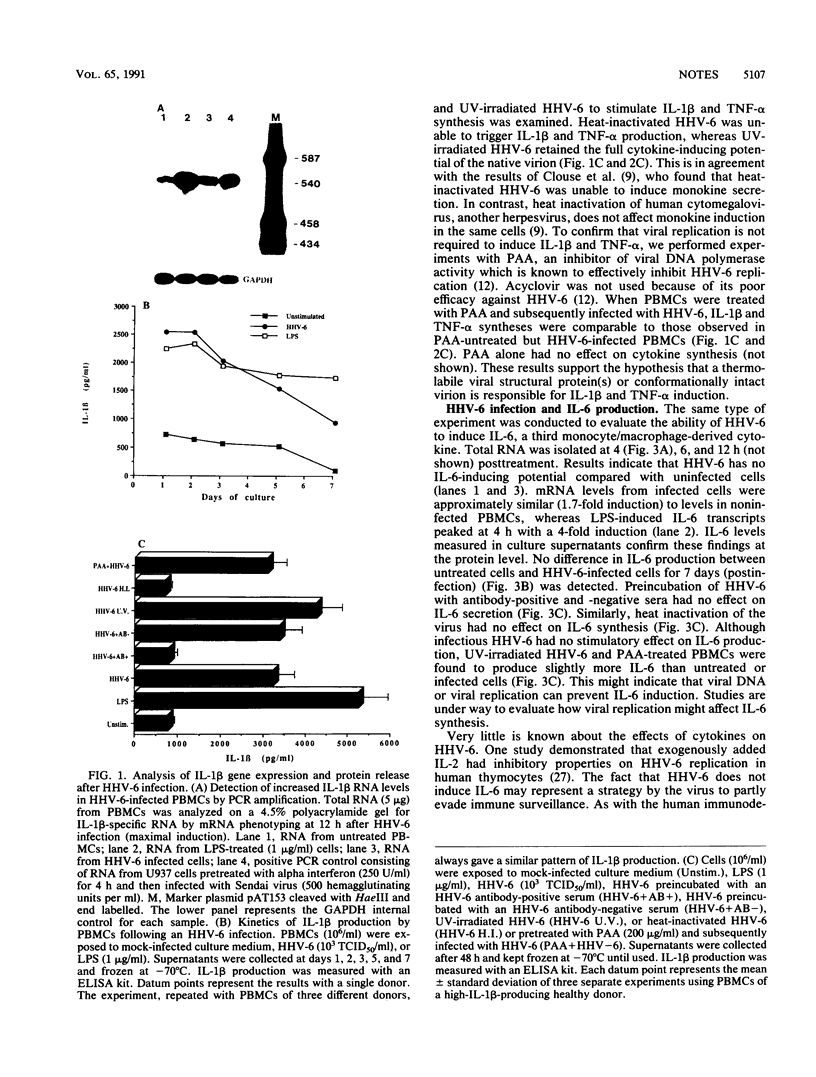
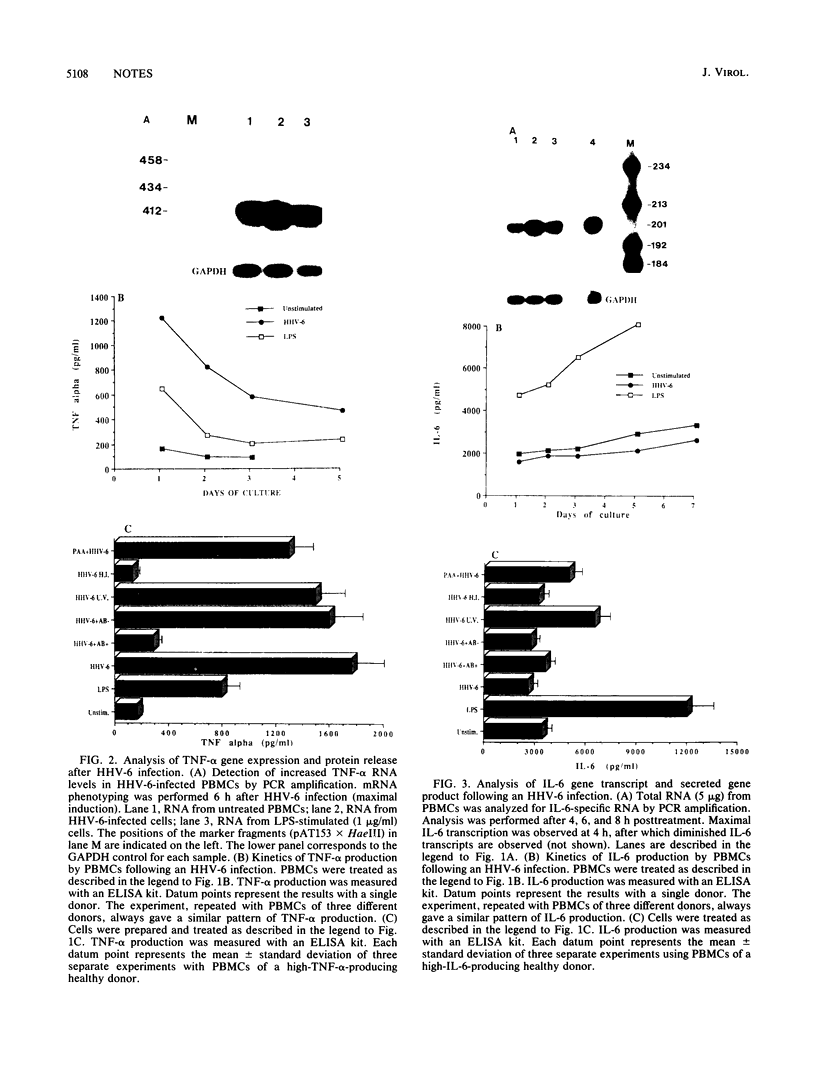
The human herpesvirus 6 (HHV-6) is known to interact intimately with cells of the immune system. Here we report that HHV-6 is a potent inducer of interleukin-1 beta (IL-1 beta) and tumor necrosis factor alpha (TNF-alpha) in cultures of peripheral blood mononuclear cells. In contradistinction, HHV-6 has no effect on IL-6 synthesis. Maximal IL-1 beta and TNF-alpha gene transcription, as detected by polymerase chain reaction amplification analysis, is observed at 12 and 6 h postinfection, respectively. Release of IL-1 beta and TNF-alpha into the culture supernatants peaked at 24 h and gradually decreased with time. Heat-inactivated virus was unable to stimulate IL-1 beta and TNF-alpha syntheses, whereas UV-irradiated virus retained the full monokine-inducing potential of the native particle. Preincubation of viral preparation with neutralizing anti-HHV-6 antibody resulted in the abrogation of this cytokine-inducing effect, whereas treatment of cells with phosphonoacetic acid (an inhibitor of viral DNA polymerase activity) had no effect on the ability of the virus to stimulate monokine release. These results indicate that HHV-6 can exert a strong immunomodulatory effect by stimulating the cells of myeloid lineage to produce these cytokines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablashi D. V., Lusso P., Hung C. L., Salahuddin S. Z., Josephs S. F., Llana T., Kramarsky B., Biberfeld P., Markham P. D., Gallo R. C. Utilization of human hematopoietic cell lines for the propagation and characterization of HBLV (human herpesvirus 6). Int J Cancer. 1988 Nov 15;42(5):787–791. doi: 10.1002/ijc.2910420526. [DOI] [PubMed] [Google Scholar]
- Ablashi D. V., Salahuddin S. Z., Josephs S. F., Imam F., Lusso P., Gallo R. C., Hung C., Lemp J., Markham P. D. HBLV (or HHV-6) in human cell lines. Nature. 1987 Sep 17;329(6136):207–207. doi: 10.1038/329207a0. [DOI] [PubMed] [Google Scholar]
- Arcari P., Martinelli R., Salvatore F. The complete sequence of a full length cDNA for human liver glyceraldehyde-3-phosphate dehydrogenase: evidence for multiple mRNA species. Nucleic Acids Res. 1984 Dec 11;12(23):9179–9189. doi: 10.1093/nar/12.23.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Briggs M., Fox J., Tedder R. S. Age prevalence of antibody to human herpesvirus 6. Lancet. 1988 May 7;1(8593):1058–1059. doi: 10.1016/s0140-6736(88)91883-1. [DOI] [PubMed] [Google Scholar]
- Buchbinder A., Josephs S. F., Ablashi D., Salahuddin S. Z., Klotman M. E., Manak M., Krueger G. R., Wong-Staal F., Gallo R. C. Polymerase chain reaction amplification and in situ hybridization for the detection of human B-lymphotropic virus. J Virol Methods. 1988 Sep;21(1-4):191–197. doi: 10.1016/0166-0934(88)90065-1. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark D. A., Alexander F. E., McKinney P. A., Roberts B. E., O'Brien C., Jarrett R. F., Cartwright R. A., Onions D. E. The seroepidemiology of human herpesvirus-6 (HHV-6) from a case-control study of leukaemia and lymphoma. Int J Cancer. 1990 May 15;45(5):829–833. doi: 10.1002/ijc.2910450507. [DOI] [PubMed] [Google Scholar]
- Clouse K. A., Robbins P. B., Fernie B., Ostrove J. M., Fauci A. S. Viral antigen stimulation of the production of human monokines capable of regulating HIV1 expression. J Immunol. 1989 Jul 15;143(2):470–475. [PubMed] [Google Scholar]
- Cordingley F. T., Bianchi A., Hoffbrand A. V., Reittie J. E., Heslop H. E., Vyakarnam A., Turner M., Meager A., Brenner M. K. Tumour necrosis factor as an autocrine tumour growth factor for chronic B-cell malignancies. Lancet. 1988 Apr 30;1(8592):969–971. doi: 10.1016/s0140-6736(88)91782-5. [DOI] [PubMed] [Google Scholar]
- D'Addario M., Roulston A., Wainberg M. A., Hiscott J. Coordinate enhancement of cytokine gene expression in human immunodeficiency virus type 1-infected promonocytic cells. J Virol. 1990 Dec;64(12):6080–6089. doi: 10.1128/jvi.64.12.6080-6089.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Luca D., Katsafanas G., Schirmer E. C., Balachandran N., Frenkel N. The replication of viral and cellular DNA in human herpesvirus 6-infected cells. Virology. 1990 Mar;175(1):199–210. doi: 10.1016/0042-6822(90)90200-b. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Clouse K. A., Justement J., Rabson A., Duh E., Kehrl J. H., Fauci A. S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. D., Briggs M., Ward P. A., Tedder R. S. Human herpesvirus 6 in salivary glands. Lancet. 1990 Sep 8;336(8715):590–593. doi: 10.1016/0140-6736(90)93392-3. [DOI] [PubMed] [Google Scholar]
- Gosselin J., Menezes J., D'Addario M., Hiscott J., Flamand L., Lamoureux G., Oth D. Inhibition of tumor necrosis factor-alpha transcription by Epstein-Barr virus. Eur J Immunol. 1991 Jan;21(1):203–208. doi: 10.1002/eji.1830210130. [DOI] [PubMed] [Google Scholar]
- Gosselin J., Menezes J., Mercier G., Lamoureux G., Oth D. Differential interleukin-2 and interferon-gamma production by human lymphocyte cultures exceptionally resistant to Epstein-Barr virus immortalization. Cell Immunol. 1989 Sep;122(2):440–449. doi: 10.1016/0008-8749(89)90090-7. [DOI] [PubMed] [Google Scholar]
- Harnett G. B., Farr T. J., Pietroboni G. R., Bucens M. R. Frequent shedding of human herpesvirus 6 in saliva. J Med Virol. 1990 Feb;30(2):128–130. doi: 10.1002/jmv.1890300209. [DOI] [PubMed] [Google Scholar]
- Josephs S. F., Salahuddin S. Z., Ablashi D. V., Schachter F., Wong-Staal F., Gallo R. C. Genomic analysis of the human B-lymphotropic virus (HBLV). Science. 1986 Oct 31;234(4776):601–603. doi: 10.1126/science.3020691. [DOI] [PubMed] [Google Scholar]
- Kikuta H., Nakane A., Lu H., Taguchi Y., Minagawa T., Matsumoto S. Interferon induction by human herpesvirus 6 in human mononuclear cells. J Infect Dis. 1990 Jul;162(1):35–38. doi: 10.1093/infdis/162.1.35. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989 Jul;74(1):1–10. [PubMed] [Google Scholar]
- Krueger G. R., Koch B., Ramon A., Ablashi D. V., Salahuddin S. Z., Josephs S. F., Streicher H. Z., Gallo R. C., Habermann U. Antibody prevalence to HBLV (human herpesvirus-6, HHV-6) and suggestive pathogenicity in the general population and in patients with immune deficiency syndromes. J Virol Methods. 1988 Sep;21(1-4):125–131. doi: 10.1016/0166-0934(88)90059-6. [DOI] [PubMed] [Google Scholar]
- Krueger G. R., Manak M., Bourgeois N., Ablashi D. V., Salahuddin S. Z., Josephs S. S., Buchbinder A., Gallo R. C., Berthold F., Tesch H. Persistent active herpes virus infection associated with atypical polyclonal lymphoproliferation (APL) and malignant lymphoma. Anticancer Res. 1989 Nov-Dec;9(6):1457–1476. [PubMed] [Google Scholar]
- Levy J. A., Ferro F., Lennette E. T., Oshiro L., Poulin L. Characterization of a new strain of HHV-6 (HHV-6SF) recovered from the saliva of an HIV-infected individual. Virology. 1990 Sep;178(1):113–121. doi: 10.1016/0042-6822(90)90384-4. [DOI] [PubMed] [Google Scholar]
- Lusso P., Markham P. D., Tschachler E., di Marzo Veronese F., Salahuddin S. Z., Ablashi D. V., Pahwa S., Krohn K., Gallo R. C. In vitro cellular tropism of human B-lymphotropic virus (human herpesvirus-6). J Exp Med. 1988 May 1;167(5):1659–1670. doi: 10.1084/jem.167.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T., Takahashi K., Balachandra K., Shiraki K., Yamanishi K., Takahashi M., Baba K. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J Clin Microbiol. 1989 Apr;27(4):651–653. doi: 10.1128/jcm.27.4.651-653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman E., Frenkel N. Interleukin-2 inhibits the replication of human herpesvirus-6 in mature thymocytes. Virology. 1990 Apr;175(2):591–594. doi: 10.1016/0042-6822(90)90447-y. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Ablashi D. V., Markham P. D., Josephs S. F., Sturzenegger S., Kaplan M., Halligan G., Biberfeld P., Wong-Staal F., Kramarsky B. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986 Oct 31;234(4776):596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- Saxinger C., Polesky H., Eby N., Grufferman S., Murphy R., Tegtmeir G., Parekh V., Memon S., Hung C. Antibody reactivity with HBLV (HHV-6) in U.S. populations. J Virol Methods. 1988 Sep;21(1-4):199–208. doi: 10.1016/0166-0934(88)90066-3. [DOI] [PubMed] [Google Scholar]
- Saxne T., Palladino M. A., Jr, Heinegård D., Talal N., Wollheim F. A. Detection of tumor necrosis factor alpha but not tumor necrosis factor beta in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum. 1988 Aug;31(8):1041–1045. doi: 10.1002/art.1780310816. [DOI] [PubMed] [Google Scholar]
- Steeper T. A., Horwitz C. A., Ablashi D. V., Salahuddin S. Z., Saxinger C., Saltzman R., Schwartz B. The spectrum of clinical and laboratory findings resulting from human herpesvirus-6 (HHV-6) in patients with mononucleosis-like illnesses not resulting from Epstein-Barr virus or cytomegalovirus. Am J Clin Pathol. 1990 Jun;93(6):776–783. doi: 10.1093/ajcp/93.6.776. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Sonoda S., Kawakami K., Miyata K., Oki T., Nagata T., Okuno T., Kamanishi K. Human herpesvirus 6 and exanthem subitum. Lancet. 1988 Jun 25;1(8600):1463–1463. doi: 10.1016/s0140-6736(88)92275-1. [DOI] [PubMed] [Google Scholar]
- Waage A., Kaufmann C., Espevik T., Husby G. Interleukin-6 in synovial fluid from patients with arthritis. Clin Immunol Immunopathol. 1989 Mar;50(3):394–398. doi: 10.1016/0090-1229(89)90146-3. [DOI] [PubMed] [Google Scholar]
- Wood D. D., Ihrie E. J., Dinarello C. A., Cohen P. L. Isolation of an interleukin-1-like factor from human joint effusions. Arthritis Rheum. 1983 Aug;26(8):975–983. doi: 10.1002/art.1780260806. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Okuno T., Shiraki K., Takahashi M., Kondo T., Asano Y., Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988 May 14;1(8594):1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]