Abstract
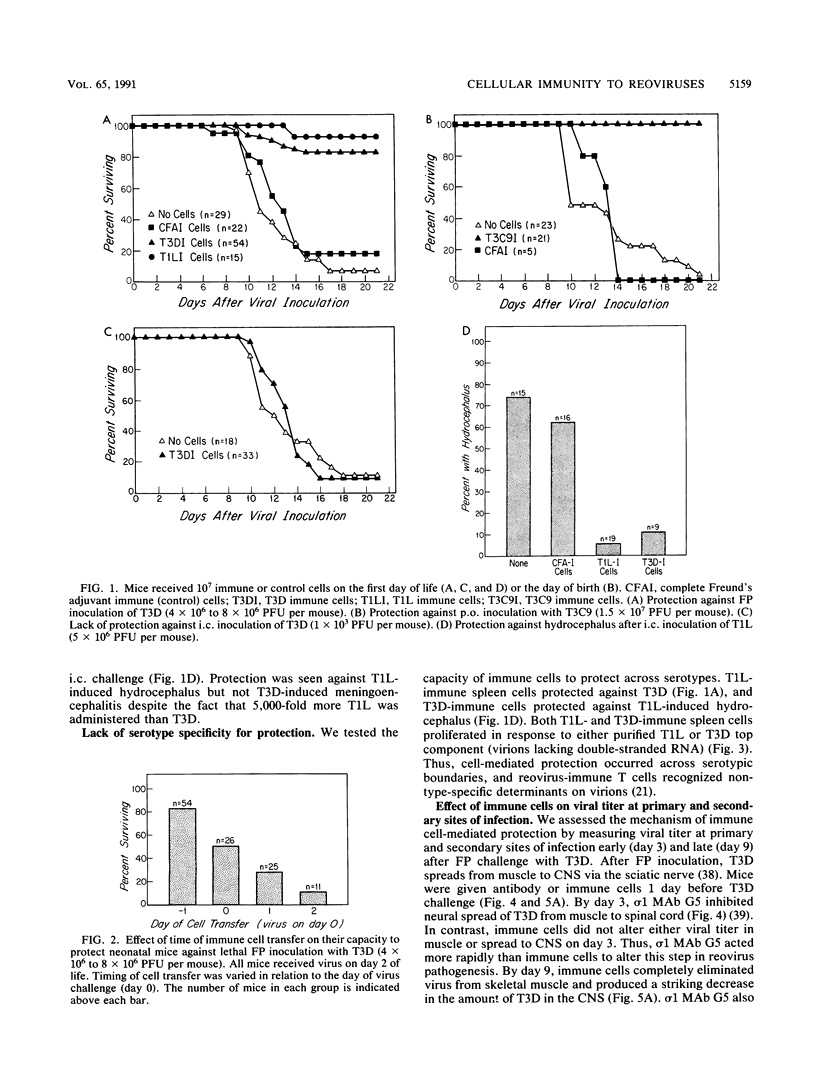
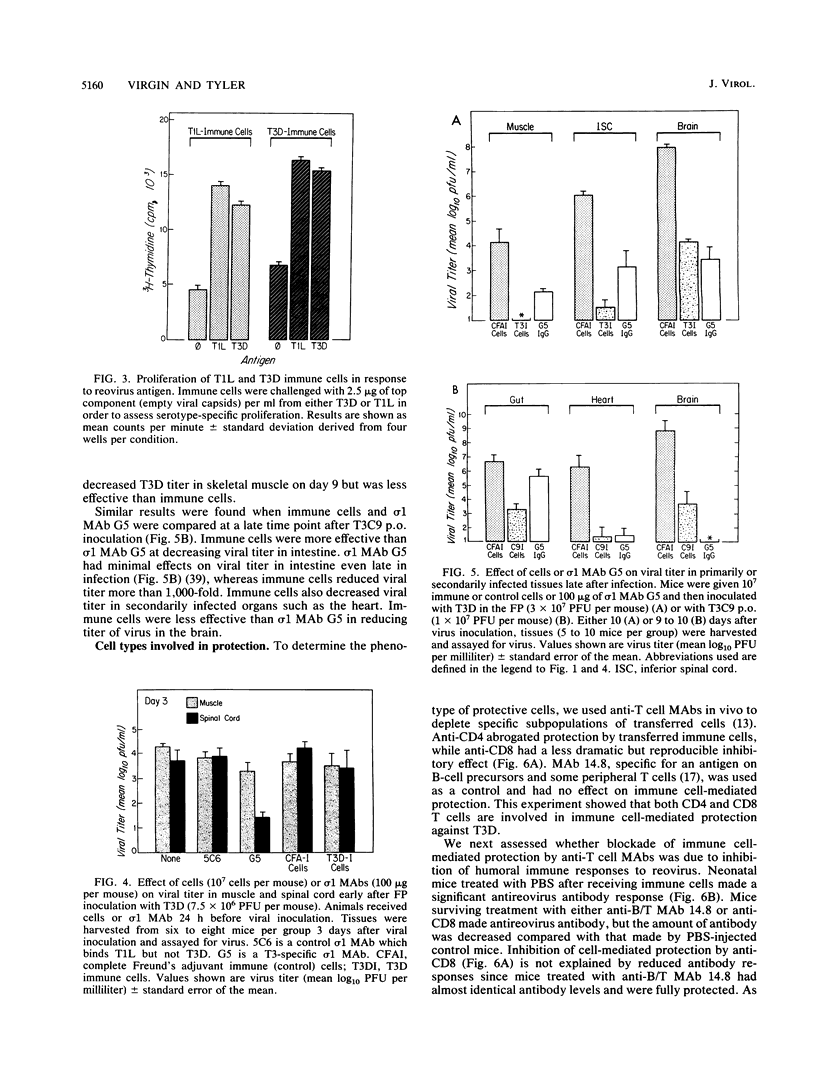
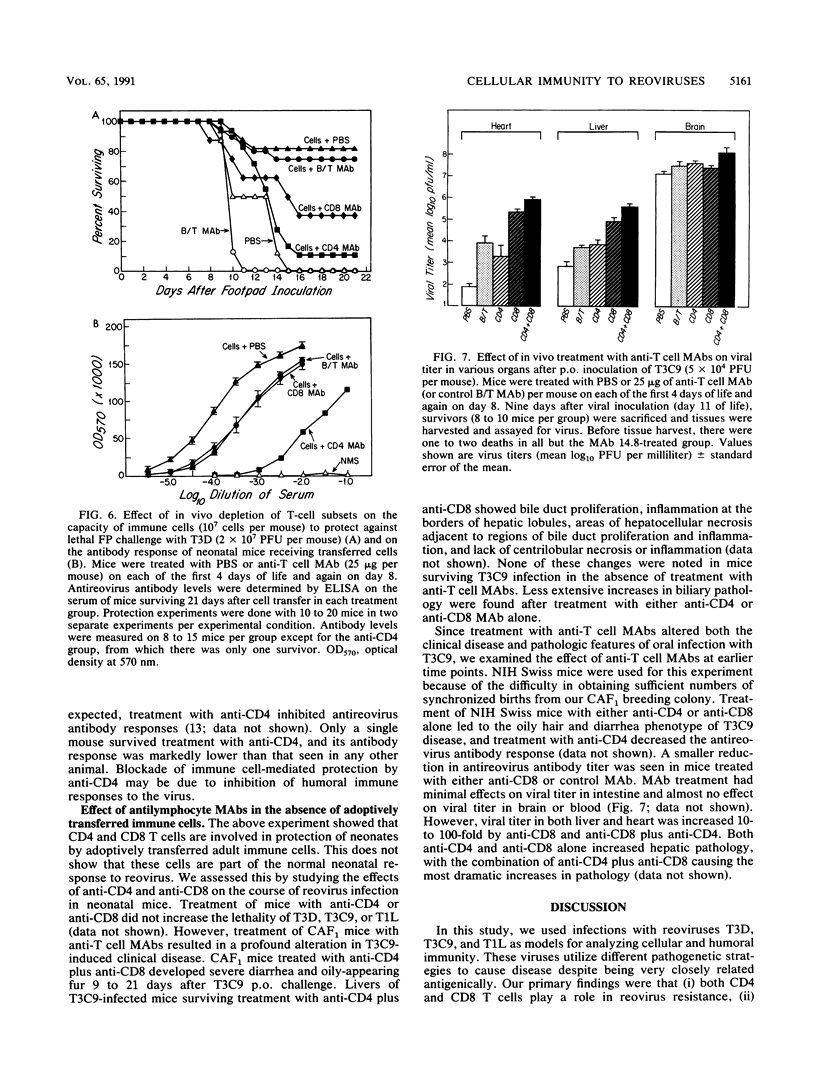
We studied the role of T cells in resistance to reovirus intestinal and central nervous system infection. Transfer of reovirus-immune adult spleen cells protected neonatal mice from (i) lethal infection with reovirus serotype 3 Dearing (T3D, footpad inoculation) and serotype 3 clone 9 (T3C9, oral inoculation) and (ii) hydrocephalus caused by serotype 1 Lang (T1L, intracranial [i.c.] inoculation). Cell-mediated protection was not serotype specific. While immune cells protected against T1L i.c., they failed to protect against 1/5,000 of the dose of T3D i.c. Two types of experiments showed that both CD4 and CD8 T cells are involved in reovirus resistance. First, immune cell-mediated protection against T3D was abrogated by in vivo treatment with anti-CD4 monoclonal antibody (MAb) and significantly inhibited by in vivo treatment with anti-CD8 MAb. Second, T3C9-infected neonatal mice treated with anti-CD4 and/or anti-CD8 developed a novel disease phenotype, an oily hair syndrome, associated with severe hepatobiliary pathology and increased viral titer in heart and liver. Immune cells and an MAb to the cell attachment protein sigma 1 (MAb G5) protected by different mechanisms. Immune cells were more effective than sigma 1 MAb G5 at controlling primary replication, while sigma 1 MAb G5 was more effective than immune cells at inhibiting neural spread of virus. We conclude that both CD4 and CD8 T cells are important for reovirus resistance, that cells and antibody act preferentially at different stages in pathogenesis in vivo, and that adoptively transferred immune cells can protect both the central nervous system and intestine.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Butler L. D., Bhatti L. T4+ T helper cell function in vivo: differential requirement for induction of antiviral cytotoxic T-cell and antibody responses. J Virol. 1988 Jun;62(6):2102–2106. doi: 10.1128/jvi.62.6.2102-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Jamieson B. D., Porter D. D. Immune therapy of a persistent and disseminated viral infection. J Virol. 1987 Dec;61(12):3920–3929. doi: 10.1128/jvi.61.12.3920-3929.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstin S. J., Spriggs D. R., Fields B. N. Evidence for functional domains on the reovirus type 3 hemagglutinin. Virology. 1982 Feb;117(1):146–155. doi: 10.1016/0042-6822(82)90514-1. [DOI] [PubMed] [Google Scholar]
- Chin T. D. Immunity induced by inactivated poliovirus vaccine and excretion of virus. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S369–S370. doi: 10.1093/clinids/6.supplement_2.s369. [DOI] [PubMed] [Google Scholar]
- Cuff C. F., Lavi E., Cebra C. K., Cebra J. J., Rubin D. H. Passive immunity to fatal reovirus serotype 3-induced meningoencephalitis mediated by both secretory and transplacental factors in neonatal mice. J Virol. 1990 Mar;64(3):1256–1263. doi: 10.1128/jvi.64.3.1256-1263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Finberg R., Spriggs D. R., Fields B. N. Host immune response to reovirus: CTL recognize the major neutralization domain of the viral hemagglutinin. J Immunol. 1982 Nov;129(5):2235–2238. [PubMed] [Google Scholar]
- Finberg R., Weiner H. L., Fields B. N., Benacerraf B., Burakoff S. J. Generation of cytolytic T lymphocytes after reovirus infection: role of S1 gene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):442–446. doi: 10.1073/pnas.76.1.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. P. Modes of action of poliovirus vaccines and relation to resulting immunity. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S352–S355. doi: 10.1093/clinids/6.supplement_2.s352. [DOI] [PubMed] [Google Scholar]
- Gaillard R. K., Jr, Joklik W. K. Quantitation of the relatedness of reovirus serotypes 1, 2, and 3 at the gene level. Virology. 1982 Nov;123(1):152–164. doi: 10.1016/0042-6822(82)90302-6. [DOI] [PubMed] [Google Scholar]
- Gaulton G. N., Sharpe A. H., Chang D. W., Fields B. N., Greene M. I. Syngeneic monoclonal internal image anti-idiotopes as prophylactic vaccines. J Immunol. 1986 Nov 1;137(9):2930–2936. [PubMed] [Google Scholar]
- George A., Kost S. I., Witzleben C. L., Cebra J. J., Rubin D. H. Reovirus-induced liver disease in severe combined immunodeficient (SCID) mice. A model for the study of viral infection, pathogenesis, and clearance. J Exp Med. 1990 Mar 1;171(3):929–934. doi: 10.1084/jem.171.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy J., Weyand C. M., Fathman C. G. Long-term humoral unresponsiveness in vivo, induced by treatment with monoclonal antibody against L3T4. J Exp Med. 1986 Sep 1;164(3):911–925. doi: 10.1084/jem.164.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes E. L., Taylor W., Mitchison N. A., Simpson E. MHC matching shows that at least two T-cell subsets determine resistance to HSV. Nature. 1979 Jan 4;277(5691):66–68. doi: 10.1038/277067a0. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Bronkhorst A. M., de Waal L. P., Melief C. J. Cooperation between cytotoxic and helper T lymphocytes in protection against lethal Sendai virus infection. Protection by T cells is MHC-restricted and MHC-regulated; a model for MHC-disease associations. J Exp Med. 1986 Sep 1;164(3):723–738. doi: 10.1084/jem.164.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilham L., Margolis G. Hydrocephalus in hamsters, ferrets, rats, and mice following inoculations with reovirus type I. I. Virologic studies. Lab Invest. 1969 Sep;21(3):183–188. [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Watanabe T., Sun L., Scheid M. P. Antigens displayed on murine B lymphocyte precursors. J Immunol. 1981 Dec;127(6):2262–2268. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lee P. W., Hayes E. C., Joklik W. K. Protein sigma 1 is the reovirus cell attachment protein. Virology. 1981 Jan 15;108(1):156–163. doi: 10.1016/0042-6822(81)90535-3. [DOI] [PubMed] [Google Scholar]
- Lightman S., Cobbold S., Waldmann H., Askonas B. A. Do L3T4+ T cells act as effector cells in protection against influenza virus infection. Immunology. 1987 Sep;62(1):139–144. [PMC free article] [PubMed] [Google Scholar]
- London S. D., Cebra J. J., Rubin D. H. The reovirus-specific cytotoxic T cell response is not restricted to serotypically unique epitopes associated with the virus hemagglutinin. Microb Pathog. 1989 Jan;6(1):43–50. doi: 10.1016/0882-4010(89)90006-5. [DOI] [PubMed] [Google Scholar]
- Lukacher A. E., Braciale V. L., Braciale T. J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984 Sep 1;160(3):814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacher A. E., Morrison L. A., Braciale V. L., Malissen B., Braciale T. J. Expression of specific cytolytic activity by H-2I region-restricted, influenza virus-specific T lymphocyte clones. J Exp Med. 1985 Jul 1;162(1):171–187. doi: 10.1084/jem.162.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch F., Doherty P. C., Ceredig R. Phenotypic and functional analysis of the cellular response in regional lymphoid tissue during an acute virus infection. J Immunol. 1989 May 15;142(10):3592–3598. [PubMed] [Google Scholar]
- Nash A. A., Jayasuriya A., Phelan J., Cobbold S. P., Waldmann H., Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987 Mar;68(Pt 3):825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- Ogra P. L. Mucosal immune response to poliovirus vaccines in childhood. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S361–S368. doi: 10.1093/clinids/6.supplement_2.s361. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Blount P., Southern P. J., Lampert P. W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986 May 15;321(6067):239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- ROSEN L. Serologic grouping of reoviruses by hemagglutination-inhibition. Am J Hyg. 1960 Mar;71:242–249. doi: 10.1093/oxfordjournals.aje.a120107. [DOI] [PubMed] [Google Scholar]
- Rubin D. H., Fields B. N. Molecular basis of reovirus virulence. Role of the M2 gene. J Exp Med. 1980 Oct 1;152(4):853–868. doi: 10.1084/jem.152.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANLEY N. F., LEAK P. J., WALTERS M. N., JOSKE R. A. MURINE INFECTIONS WITH REOVIRUS. II. THE CHRONIC DISEASE FOLLOWING REOVIRUS TYPE 3 INFECTION. Br J Exp Pathol. 1964 Apr;45:142–149. [PMC free article] [PubMed] [Google Scholar]
- STNALEY N. F., LEAK P. J. MURINE INFECTION WITH REOVIRUS TYPE 3 AND THE RUNTING SYNDROME. Nature. 1963 Sep 28;199:1309–1310. doi: 10.1038/1991309a0. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Gaulton G. N., McDade K. K., Fields B. N., Greene M. I. Syngeneic monoclonal antiidiotype can induce cellular immunity to reovirus. J Exp Med. 1984 Oct 1;160(4):1195–1205. doi: 10.1084/jem.160.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Sussman M. A., Shubin R. A., Kyuwa S., Stohlman S. A. T-cell-mediated clearance of mouse hepatitis virus strain JHM from the central nervous system. J Virol. 1989 Jul;63(7):3051–3056. doi: 10.1128/jvi.63.7.3051-3056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu M., Powers M. L., Weiner H. L. Age dependent susceptibility to Reovirus type 3 encephalitis: role of viral and host factors. Ann Neurol. 1983 Jun;13(6):602–607. doi: 10.1002/ana.410130604. [DOI] [PubMed] [Google Scholar]
- Tyler K. L., McPhee D. A., Fields B. N. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science. 1986 Aug 15;233(4765):770–774. doi: 10.1126/science.3016895. [DOI] [PubMed] [Google Scholar]
- Tyler K. L., Virgin H. W., 4th, Bassel-Duby R., Fields B. N. Antibody inhibits defined stages in the pathogenesis of reovirus serotype 3 infection of the central nervous system. J Exp Med. 1989 Sep 1;170(3):887–900. doi: 10.1084/jem.170.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Bassel-Duby R., Fields B. N., Tyler K. L. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J Virol. 1988 Dec;62(12):4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTERS M. N., LEAK P. J., JOSKE R. A., STANLEY N. F., PERRET D. H. MURINE INFECTION WITH REOVIRUS. 3. PATHOLOGY OF INFECTION WITH TYPES 1 AND 2. Br J Exp Pathol. 1965 Apr;46:200–212. [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Drayna D., Averill D. R., Jr, Fields B. N. Molecular basis of reovirus virulence: role of the S1 gene. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5744–5748. doi: 10.1073/pnas.74.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Fields B. N. Neutralization of reovirus: the gene responsible for the neutralization antigen. J Exp Med. 1977 Nov 1;146(5):1305–1310. doi: 10.1084/jem.146.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Powers M. L., Fields B. N. Absolute linkage of virulence and central nervous system cell tropism of reoviruses to viral hemagglutinin. J Infect Dis. 1980 May;141(5):609–616. doi: 10.1093/infdis/141.5.609. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Ramig R. F., Mustoe T. A., Fields B. N. Identification of the gene coding for the hemagglutinin of reovirus. Virology. 1978 May 15;86(2):581–584. doi: 10.1016/0042-6822(78)90099-5. [DOI] [PubMed] [Google Scholar]
- Williams W. V., London S. D., Weiner D. B., Wadsworth S., Berzofsky J. A., Robey F., Rubin D. H., Greene M. I. Immune response to a molecularly defined internal image idiotope. J Immunol. 1989 Jun 15;142(12):4392–4400. [PubMed] [Google Scholar]


