Abstract
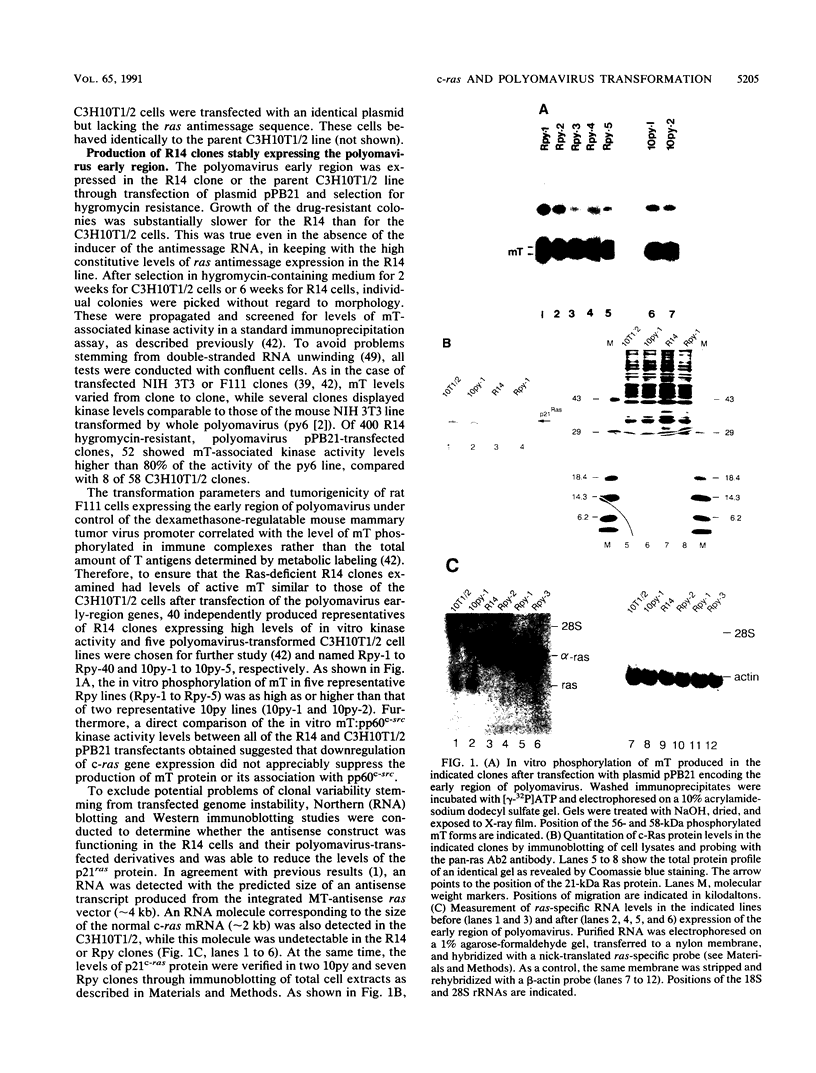
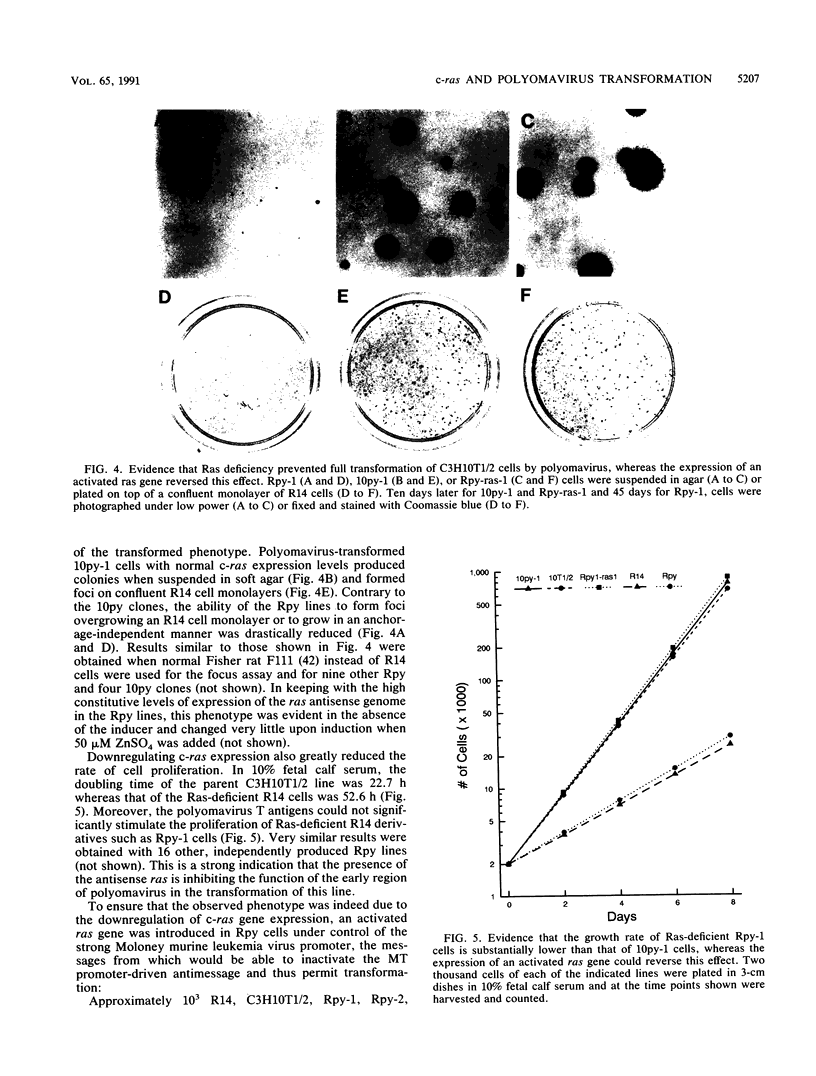
To investigate the role of ras gene activity in cellular transformation by polyomavirus, murine C3H10T1/2 cells were rendered ras deficient by transfection with an antisense ras gene construct. Ras deficiency resulted in a partial suppression of the polyomavirus-induced transformed phenotype. The production of viral middle T antigen and its association with pp60c-src, increased membrane-associated protein kinase C activity, and morphological transformation were unaffected by the downregulation of c-ras gene expression. On the other hand, stimulated proliferation, focus formation on confluent monolayers of normal cells, and colony formation in soft agar were all greatly reduced in cells containing reduced p21ras levels. It is concluded that ras gene activity is needed for full cell transformation by polyomavirus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. K., Stankova J., Roder J. C. Decreased p21 levels in anti-sense ras transfectants augments NK sensitivity. Mol Immunol. 1989 Oct;26(10):985–991. doi: 10.1016/0161-5890(89)90117-x. [DOI] [PubMed] [Google Scholar]
- Benjamin T. L. Host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1970 Sep;67(1):394–399. doi: 10.1073/pnas.67.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blochlinger K., Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984 Dec;4(12):2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Chakravarthy B. R., Durkin J. P., Rixon R. H., Whitfield J. F. Parathyroid hormone fragment [3-34] stimulates protein kinase C (PKC) activity in rat osteosarcoma and murine T-lymphoma cells. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1105–1110. doi: 10.1016/0006-291x(90)90798-r. [DOI] [PubMed] [Google Scholar]
- Chakravarthy B. R., Franks D. J., Whitfield J. F., Durkin J. P. A novel method for measuring protein kinase C activity in a native membrane-associated state. Biochem Biophys Res Commun. 1989 Apr 14;160(1):340–345. doi: 10.1016/0006-291x(89)91661-6. [DOI] [PubMed] [Google Scholar]
- Cheng S. H., Harvey R., Espino P. C., Semba K., Yamamoto T., Toyoshima K., Smith A. E. Peptide antibodies to the human c-fyn gene product demonstrate pp59c-fyn is capable of complex formation with the middle-T antigen of polyomavirus. EMBO J. 1988 Dec 1;7(12):3845–3855. doi: 10.1002/j.1460-2075.1988.tb03270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Markland W., Markham A. F., Smith A. E. Mutations around the NG59 lesion indicate an active association of polyoma virus middle-T antigen with pp60c-src is required for cell transformation. EMBO J. 1986 Feb;5(2):325–334. doi: 10.1002/j.1460-2075.1986.tb04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., King C. S. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol Cell Biol. 1986 Dec;6(12):4467–4477. doi: 10.1128/mcb.6.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Der C. J., Finkel T., Cooper G. M. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986 Jan 17;44(1):167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- Durkin J. P., Whitfield J. F. Characterization of the mitogenic signal from an oncogene ras protein. Anticancer Res. 1989 Sep-Oct;9(5):1313–1323. [PubMed] [Google Scholar]
- Ellis C., Moran M., McCormick F., Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990 Jan 25;343(6256):377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Frech M., John J., Pizon V., Chardin P., Tavitian A., Clark R., McCormick F., Wittinghofer A. Inhibition of GTPase activating protein stimulation of Ras-p21 GTPase by the Krev-1 gene product. Science. 1990 Jul 13;249(4965):169–171. doi: 10.1126/science.2164710. [DOI] [PubMed] [Google Scholar]
- Gélinas C., Bouchard L., Bastin M. Tumorigenic activity of cloned polyoma virus DNA in newborn rats. Experientia. 1981 Oct 15;37(10):1074–1075. doi: 10.1007/BF02085017. [DOI] [PubMed] [Google Scholar]
- Hunter T. A tail of two src's: mutatis mutandis. Cell. 1987 Apr 10;49(1):1–4. doi: 10.1016/0092-8674(87)90745-8. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Morrison D. K., Wong G., McCormick F., Williams L. T. PDGF beta-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell. 1990 Apr 6;61(1):125–133. doi: 10.1016/0092-8674(90)90220-9. [DOI] [PubMed] [Google Scholar]
- Kaplan P. L., Ozanne B. Polyoma virus-transformed cells produce transforming growth factor(s) and grow in serum-free medium. Virology. 1982 Dec;123(2):372–380. doi: 10.1016/0042-6822(82)90270-7. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Ellis C., Pawson T., Cooper J. A. Binding of GAP to activated PDGF receptors. Science. 1990 Mar 30;247(4950):1578–1581. doi: 10.1126/science.2157284. [DOI] [PubMed] [Google Scholar]
- Kitayama H., Sugimoto Y., Matsuzaki T., Ikawa Y., Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989 Jan 13;56(1):77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Frantz J. D., Strominger J. L., Mulligan R. C. Expression of human class II major histocompatibility complex antigens using retrovirus vectors. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2150–2154. doi: 10.1073/pnas.84.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth S., Sudol M., Hanafusa H. Association of the polyomavirus middle-T antigen with c-yes protein. Nature. 1987 Jan 8;325(7000):171–173. doi: 10.1038/325171a0. [DOI] [PubMed] [Google Scholar]
- Kypta R. M., Hemming A., Courtneidge S. A. Identification and characterization of p59fyn (a src-like protein tyrosine kinase) in normal and polyoma virus transformed cells. EMBO J. 1988 Dec 1;7(12):3837–3844. doi: 10.1002/j.1460-2075.1988.tb03269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie R. R., King C. S., MacAuley A., Marth J. D., Perlmutter R. M., Eckhart W., Cooper J. A. p56lck protein-tyrosine kinase is cytoskeletal and does not bind to polyomavirus middle T antigen. J Virol. 1988 Dec;62(12):4673–4679. doi: 10.1128/jvi.62.12.4673-4679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Marcellus R., Whitfield J. F., Raptis L. Polyoma virus middle tumor antigen stimulates membrane-associated protein kinase C at lower levels than required for phosphatidylinositol kinase activation and neoplastic transformation. Oncogene. 1991 Jun;6(6):1037–1040. [PubMed] [Google Scholar]
- Marshall C. J., Lloyd A. C., Morris J. D., Paterson H., Price B., Hall A. Signal transduction by p21ras. Int J Cancer Suppl. 1989;4:29–31. doi: 10.1002/ijc.2910440708. [DOI] [PubMed] [Google Scholar]
- Matthews J. T., Benjamin T. L. 12-O-tetradecanoylphorbol-13-acetate stimulates phosphorylation of the 58,000-Mr form of polyomavirus middle T antigen in vivo: implications for a possible role of protein kinase C in middle T function. J Virol. 1986 May;58(2):239–246. doi: 10.1128/jvi.58.2.239-246.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S. A colorimetric method for the determination of submicrogram quantities of protein. Anal Biochem. 1977 Mar;78(1):86–92. doi: 10.1016/0003-2697(77)90011-2. [DOI] [PubMed] [Google Scholar]
- Molloy C. J., Bottaro D. P., Fleming T. P., Marshall M. S., Gibbs J. B., Aaronson S. A. PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature. 1989 Dec 7;342(6250):711–714. doi: 10.1038/342711a0. [DOI] [PubMed] [Google Scholar]
- Morris J. D., Price B., Lloyd A. C., Self A. J., Marshall C. J., Hall A. Scrape-loading of Swiss 3T3 cells with ras protein rapidly activates protein kinase C in the absence of phosphoinositide hydrolysis. Oncogene. 1989 Jan;4(1):27–31. [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Raptis L., Bell J., Whitfield J. F. Protein kinase C increases the activity of the polyoma virus middle T antigen-associated phosphatidylinositol kinase. Biochem Biophys Res Commun. 1988 Jul 15;154(1):306–311. doi: 10.1016/0006-291x(88)90685-7. [DOI] [PubMed] [Google Scholar]
- Raptis L., Bolen J. B. Polyomavirus transforms rat F111 and mouse NIH 3T3 cells by different mechanisms. J Virol. 1989 Feb;63(2):753–758. doi: 10.1128/jvi.63.2.753-758.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raptis L., Boynton A. L., Whitfield J. F. Protein kinase C promotes the phosphorylation of immunoprecipitated middle T antigen from polyoma-transformed cells. Biochem Biophys Res Commun. 1986 May 14;136(3):995–1000. doi: 10.1016/0006-291x(86)90431-6. [DOI] [PubMed] [Google Scholar]
- Raptis L., Firth K. L. Electroporation of adherent cells in situ. DNA Cell Biol. 1990 Oct;9(8):615–621. doi: 10.1089/dna.1990.9.615. [DOI] [PubMed] [Google Scholar]
- Raptis L., Lamfrom H., Benjamin T. L. Regulation of cellular phenotype and expression of polyomavirus middle T antigen in rat fibroblasts. Mol Cell Biol. 1985 Sep;5(9):2476–2486. doi: 10.1128/mcb.5.9.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raptis L. Polyomavirus middle tumor antigen increases responsiveness to growth factors. J Virol. 1991 May;65(5):2691–2694. doi: 10.1128/jvi.65.5.2691-2694.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaber M. D., Garsky V. M., Boylan D., Hill W. S., Scolnick E. M., Marshall M. S., Sigal I. S., Gibbs J. B. Ras interaction with the GTPase-activating protein (GAP). Proteins. 1989;6(3):306–315. doi: 10.1002/prot.340060313. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Tsai M. H., Yu C. L., Smith J. K. Critical role of cellular ras proteins in proliferative signal transduction. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):871–881. doi: 10.1101/sqb.1988.053.01.100. [DOI] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Trimble W. S., Johnson P. W., Hozumi N., Roder J. C. Inducible cellular transformation by a metallothionein-ras hybrid oncogene leads to natural killer cell susceptibility. Nature. 1986 Jun 19;321(6072):782–784. doi: 10.1038/321782a0. [DOI] [PubMed] [Google Scholar]
- Ueyama H., Hamada H., Battula N., Kakunaga T. Structure of a human smooth muscle actin gene (aortic type) with a unique intron site. Mol Cell Biol. 1984 Jun;4(6):1073–1078. doi: 10.1128/mcb.4.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. W., Nishikura K. Cell cycle expression of RNA duplex unwindase activity in mammalian cells. Mol Cell Biol. 1988 Feb;8(2):770–777. doi: 10.1128/mcb.8.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]







