Abstract
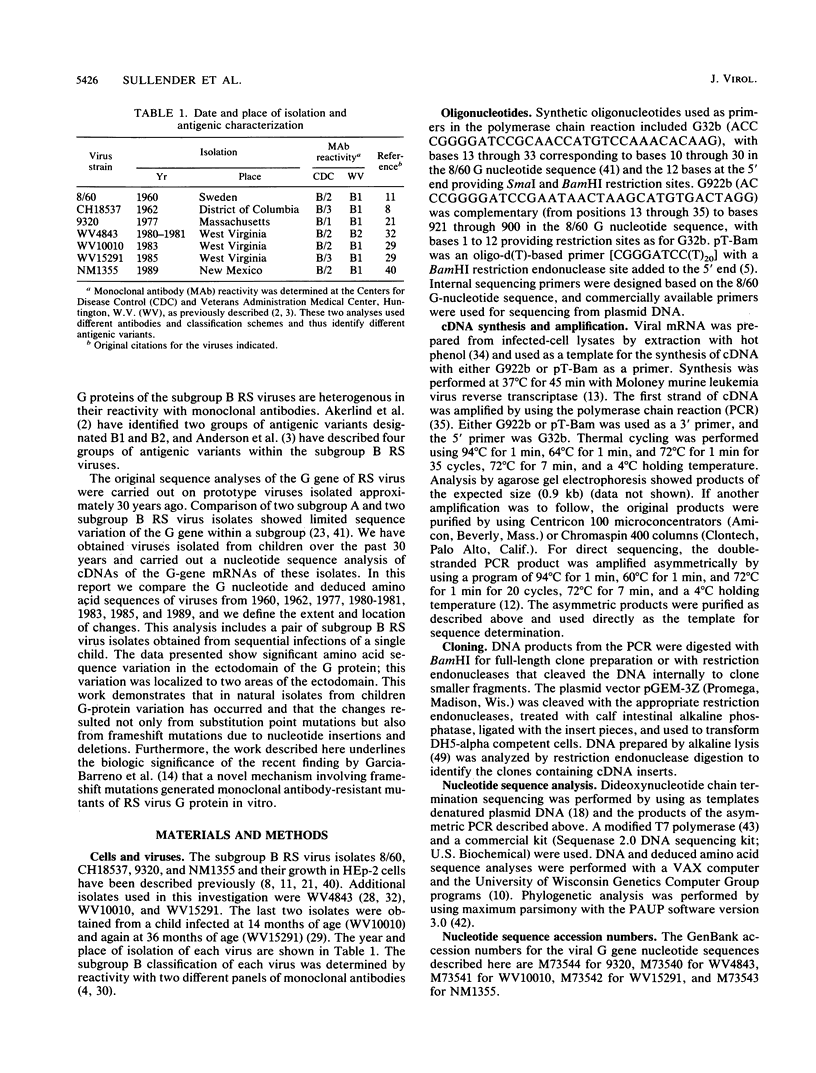
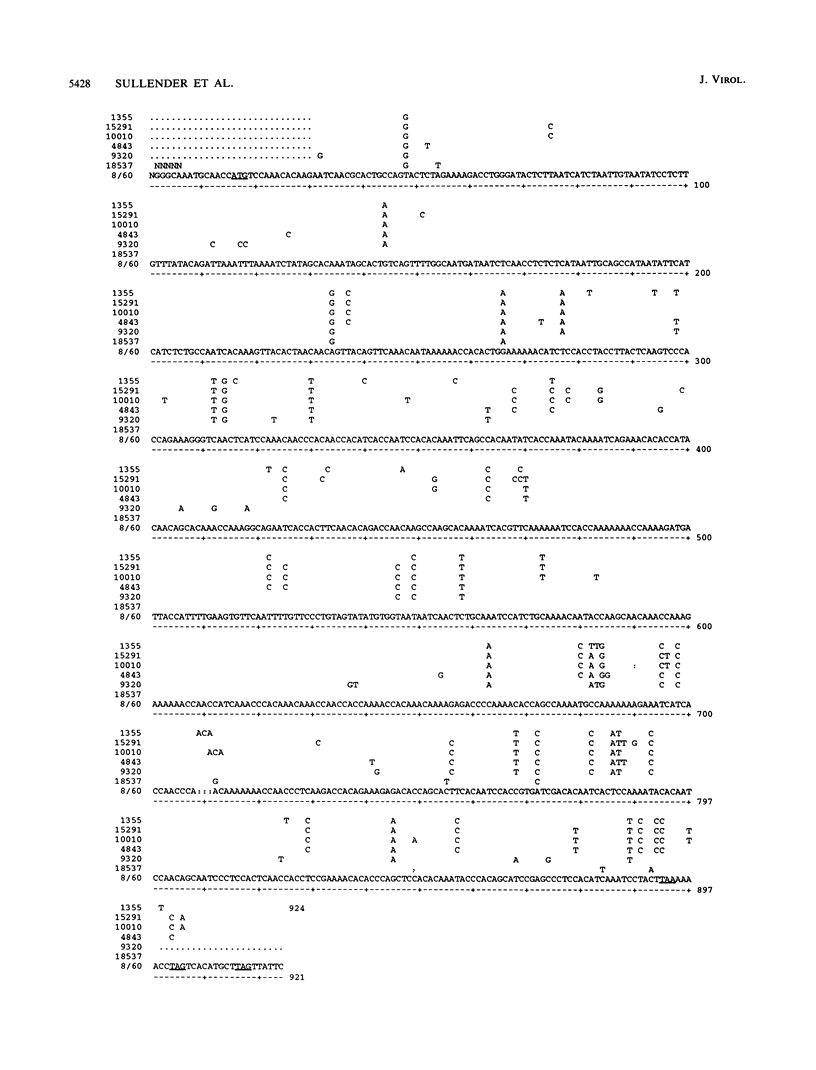
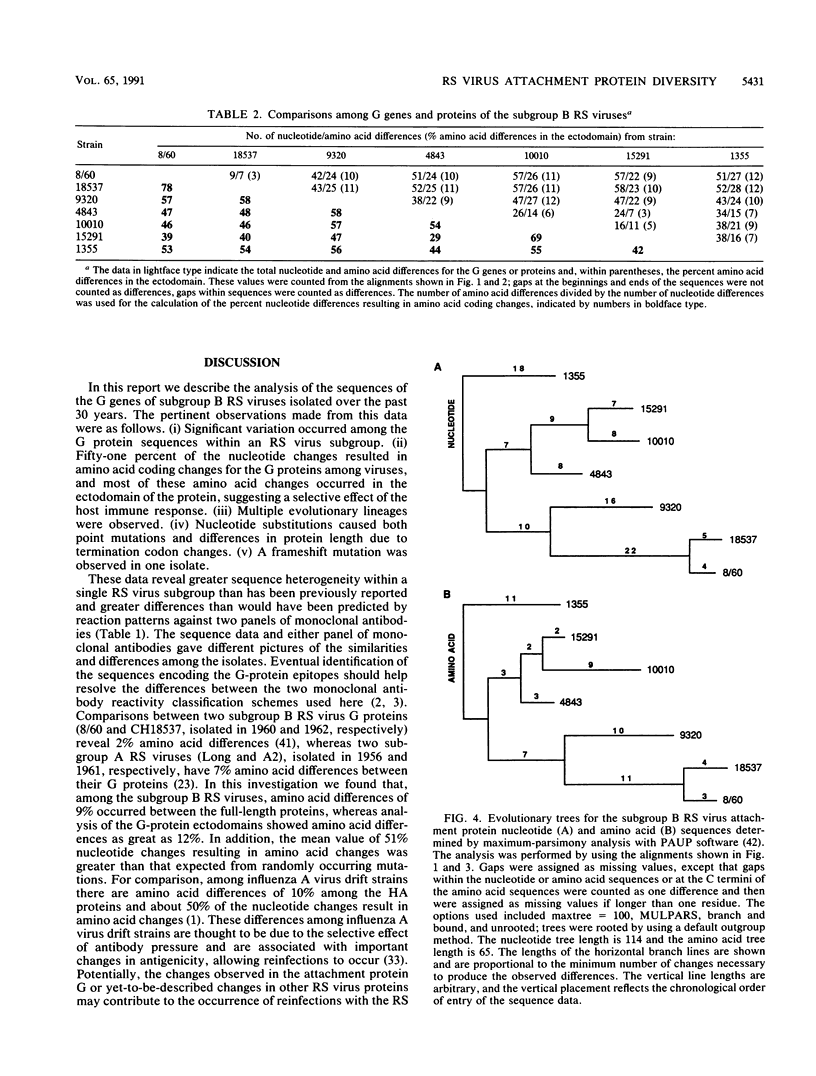
Respiratory syncytial (RS) virus causes repeated infections throughout life. Between the two main antigenic subgroups of RS virus, there is antigenic variation in the attachment protein G. The antigenic differences between the subgroups appear to play a role in allowing repeated infections to occur. Antigenic differences also occur within subgroups; however, neither the extent of these differences nor their contributions to repeat infections are known. We report a molecular analysis of the extent of diversity within the subgroup B RS virus attachment protein genes of viruses isolated from children over a 30-year period. Amino acid sequence differences as high as 12% were observed in the ectodomains of the G proteins among the isolates, whereas the cytoplasmic and transmembrane domains were highly conserved. The changes in the G-protein ectodomain were localized to two areas on either side of a highly conserved region surrounding four cysteine residues. Strikingly, single-amino-acid coding changes generated by substitution mutations were not the only means by which change occurred. Changes also occurred by (i) substitutions that changed the available termination codons, resulting in proteins of various lengths, and (ii) a mutation introduced by a single nucleotide deletion and subsequent nucleotide insertion, which caused a shift in the open reading frame of the protein in comparison to the other G genes analyzed. Fifty-one percent of the G-gene nucleotide changes observed among the isolates resulted in amino acid coding changes in the G protein, indicating a selective pressure for change. Maximum-parsimony analysis demonstrated that distinct evolutionary lineages existed. These data show that sequence diversity exists among the G proteins within the subgroup B RS viruses, and this diversity may be important in the immunobiology of the RS viruses.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M., Gibbs A. J., Laver W. G., Webster R. G. Evolutionary changes in influenza B are not primarily governed by antibody selection. Proc Natl Acad Sci U S A. 1990 May;87(10):3884–3888. doi: 10.1073/pnas.87.10.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerlind B., Norrby E., Orvell C., Mufson M. A. Respiratory syncytial virus: heterogeneity of subgroup B strains. J Gen Virol. 1988 Sep;69(Pt 9):2145–2154. doi: 10.1099/0022-1317-69-9-2145. [DOI] [PubMed] [Google Scholar]
- Anderson L. J., Hendry R. M., Pierik L. T., Tsou C., McIntosh K. Multicenter study of strains of respiratory syncytial virus. J Infect Dis. 1991 Apr;163(4):687–692. doi: 10.1093/infdis/163.4.687. [DOI] [PubMed] [Google Scholar]
- Anderson L. J., Hierholzer J. C., Tsou C., Hendry R. M., Fernie B. F., Stone Y., McIntosh K. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis. 1985 Apr;151(4):626–633. doi: 10.1093/infdis/151.4.626. [DOI] [PubMed] [Google Scholar]
- Berchtold M. W. A simple method for direct cloning and sequencing cDNA by the use of a single specific oligonucleotide and oligo(dT) in a polymerase chain reaction (PCR). Nucleic Acids Res. 1989 Jan 11;17(1):453–453. doi: 10.1093/nar/17.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COATES H. V., KENDRICK L., CHANOCK R. M. Antigenic differences between two strains of respiratory syncytial virus. Proc Soc Exp Biol Med. 1963 Apr;112:958–964. doi: 10.3181/00379727-112-28221. [DOI] [PubMed] [Google Scholar]
- Cane P. A., Pringle C. R. Respiratory syncytial virus heterogeneity during an epidemic: analysis by limited nucleotide sequencing (SH gene) and restriction mapping (N gene). J Gen Virol. 1991 Feb;72(Pt 2):349–357. doi: 10.1099/0022-1317-72-2-349. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Kaelin K., Baczko K., Billeter M. A. Measles virus editing provides an additional cysteine-rich protein. Cell. 1989 Mar 10;56(5):759–764. doi: 10.1016/0092-8674(89)90679-x. [DOI] [PubMed] [Google Scholar]
- Cristina J., López J. A., Albó C., García-Barreno B., García J., Melero J. A., Portela A. Analysis of genetic variability in human respiratory syncytial virus by the RNase A mismatch cleavage method: subtype divergence and heterogeneity. Virology. 1990 Jan;174(1):126–134. doi: 10.1016/0042-6822(90)90061-u. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett J. E., Taylor-Robinson D. Serological studies with respiratory syncytial virus. Arch Gesamte Virusforsch. 1965;15(5):601–608. doi: 10.1007/BF01245207. [DOI] [PubMed] [Google Scholar]
- Ferre F., Garduno F. Preparation of crude cell extract suitable for amplification of RNA by the polymerase chain reaction. Nucleic Acids Res. 1989 Mar 11;17(5):2141–2141. doi: 10.1093/nar/17.5.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Barreno B., Portela A., Delgado T., López J. A., Melero J. A. Frame shift mutations as a novel mechanism for the generation of neutralization resistant mutants of human respiratory syncytial virus. EMBO J. 1990 Dec;9(12):4181–4187. doi: 10.1002/j.1460-2075.1990.tb07642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen W. P., Paredes A., Allison J. E., Taber L. H., Frank A. L. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981 May;98(5):708–715. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- Gorman O. T., Donis R. O., Kawaoka Y., Webster R. G. Evolution of influenza A virus PB2 genes: implications for evolution of the ribonucleoprotein complex and origin of human influenza A virus. J Virol. 1990 Oct;64(10):4893–4902. doi: 10.1128/jvi.64.10.4893-4902.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K. C., Kingsbury D. W. Complete sequences of the intergenic and mRNA start signals in the Sendai virus genome: homologies with the genome of vesicular stomatitis virus. Nucleic Acids Res. 1984 May 11;12(9):3829–3841. doi: 10.1093/nar/12.9.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltiner M., Kempe T., Tjian R. A novel strategy for constructing clustered point mutations. Nucleic Acids Res. 1985 Feb 11;13(3):1015–1025. doi: 10.1093/nar/13.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson F. W., Collier A. M., Clyde W. A., Jr, Denny F. W. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979 Mar 8;300(10):530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- Hendry R. M., Pierik L. T., McIntosh K. Prevalence of respiratory syncytial virus subgroups over six consecutive outbreaks: 1981-1987. J Infect Dis. 1989 Aug;160(2):185–190. doi: 10.1093/infdis/160.2.185. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Hirsch M. S. Croup and pneumonia in human infants associated with a new strain of respiratory syncytial virus. J Infect Dis. 1979 Nov;140(5):826–828. doi: 10.1093/infdis/140.5.826. [DOI] [PubMed] [Google Scholar]
- Johnson P. R., Collins P. L. The A and B subgroups of human respiratory syncytial virus: comparison of intergenic and gene-overlap sequences. J Gen Virol. 1988 Nov;69(Pt 11):2901–2906. doi: 10.1099/0022-1317-69-11-2901. [DOI] [PubMed] [Google Scholar]
- Johnson P. R., Jr, Olmsted R. A., Prince G. A., Murphy B. R., Alling D. W., Walsh E. E., Collins P. L. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J Virol. 1987 Oct;61(10):3163–3166. doi: 10.1128/jvi.61.10.3163-3166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. R., Spriggs M. K., Olmsted R. A., Collins P. L. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5625–5629. doi: 10.1073/pnas.84.16.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Lerch R. A., Anderson K., Wertz G. W. Nucleotide sequence analysis and expression from recombinant vectors demonstrate that the attachment protein G of bovine respiratory syncytial virus is distinct from that of human respiratory syncytial virus. J Virol. 1990 Nov;64(11):5559–5569. doi: 10.1128/jvi.64.11.5559-5569.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson M. A., Akerlind-Stopner B., Orvell C., Belshe R. B., Norrby E. A single-season epidemic with respiratory syncytial virus subgroup B2 during 10 epidemic years, 1978 to 1988. J Clin Microbiol. 1991 Jan;29(1):162–165. doi: 10.1128/jcm.29.1.162-165.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson M. A., Belshe R. B., Orvell C., Norrby E. Subgroup characteristics of respiratory syncytial virus strains recovered from children with two consecutive infections. J Clin Microbiol. 1987 Aug;25(8):1535–1539. doi: 10.1128/jcm.25.8.1535-1539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson M. A., Orvell C., Rafnar B., Norrby E. Two distinct subtypes of human respiratory syncytial virus. J Gen Virol. 1985 Oct;66(Pt 10):2111–2124. doi: 10.1099/0022-1317-66-10-2111. [DOI] [PubMed] [Google Scholar]
- Norrby E., Mufson M. A., Sheshberadaran H. Structural differences between subtype A and B strains of respiratory syncytial virus. J Gen Virol. 1986 Dec;67(Pt 12):2721–2729. doi: 10.1099/0022-1317-67-12-2721. [DOI] [PubMed] [Google Scholar]
- Orvell C., Norrby E., Mufson M. A. Preparation and characterization of monoclonal antibodies directed against five structural components of human respiratory syncytial virus subgroup B. J Gen Virol. 1987 Dec;68(Pt 12):3125–3135. doi: 10.1099/0022-1317-68-12-3125. [DOI] [PubMed] [Google Scholar]
- Palese P., Young J. F. Variation of influenza A, B, and C viruses. Science. 1982 Mar 19;215(4539):1468–1474. doi: 10.1126/science.7038875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Storch G. A., Anderson L. J., Park C. S., Tsou C., Dohner D. E. Antigenic and genomic diversity within group A respiratory syncytial virus. J Infect Dis. 1991 Apr;163(4):858–861. doi: 10.1093/infdis/163.4.858. [DOI] [PubMed] [Google Scholar]
- Storch G. A., Park C. S., Dohner D. E. RNA fingerprinting of respiratory syncytial virus using ribonuclease protection. Application to molecular epidemiology. J Clin Invest. 1989 Jun;83(6):1894–1902. doi: 10.1172/JCI114096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Taylor G., Ball L. A., Anderson K., Young K. K., King A. M., Wertz G. W. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J Virol. 1987 Dec;61(12):3855–3861. doi: 10.1128/jvi.61.12.3855-3861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullender W. M., Anderson L. J., Anderson K., Wertz G. W. Differentiation of respiratory syncytial virus subgroups with cDNA probes in a nucleic acid hybridization assay. J Clin Microbiol. 1990 Aug;28(8):1683–1687. doi: 10.1128/jcm.28.8.1683-1687.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. M., Lamb R. A., Paterson R. G. Two mRNAs that differ by two nontemplated nucleotides encode the amino coterminal proteins P and V of the paramyxovirus SV5. Cell. 1988 Sep 9;54(6):891–902. doi: 10.1016/S0092-8674(88)91285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Curran J., Kolakofsky D. A stuttering model for paramyxovirus P mRNA editing. EMBO J. 1990 Jun;9(6):2017–2022. doi: 10.1002/j.1460-2075.1990.tb08330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Collins P. L., Huang Y., Gruber C., Levine S., Ball L. A. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4075–4079. doi: 10.1073/pnas.82.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Krieger M., Ball L. A. Structure and cell surface maturation of the attachment glycoprotein of human respiratory syncytial virus in a cell line deficient in O glycosylation. J Virol. 1989 Nov;63(11):4767–4776. doi: 10.1128/jvi.63.11.4767-4776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M., Krystal M., Fitch W. M., Palese P. Influenza B virus evolution: co-circulating lineages and comparison of evolutionary pattern with those of influenza A and C viruses. Virology. 1988 Mar;163(1):112–122. doi: 10.1016/0042-6822(88)90238-3. [DOI] [PubMed] [Google Scholar]
- Zhou C., Yang Y., Jong A. Y. Mini-prep in ten minutes. Biotechniques. 1990 Feb;8(2):172–173. [PubMed] [Google Scholar]


