Abstract
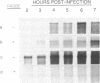
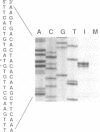
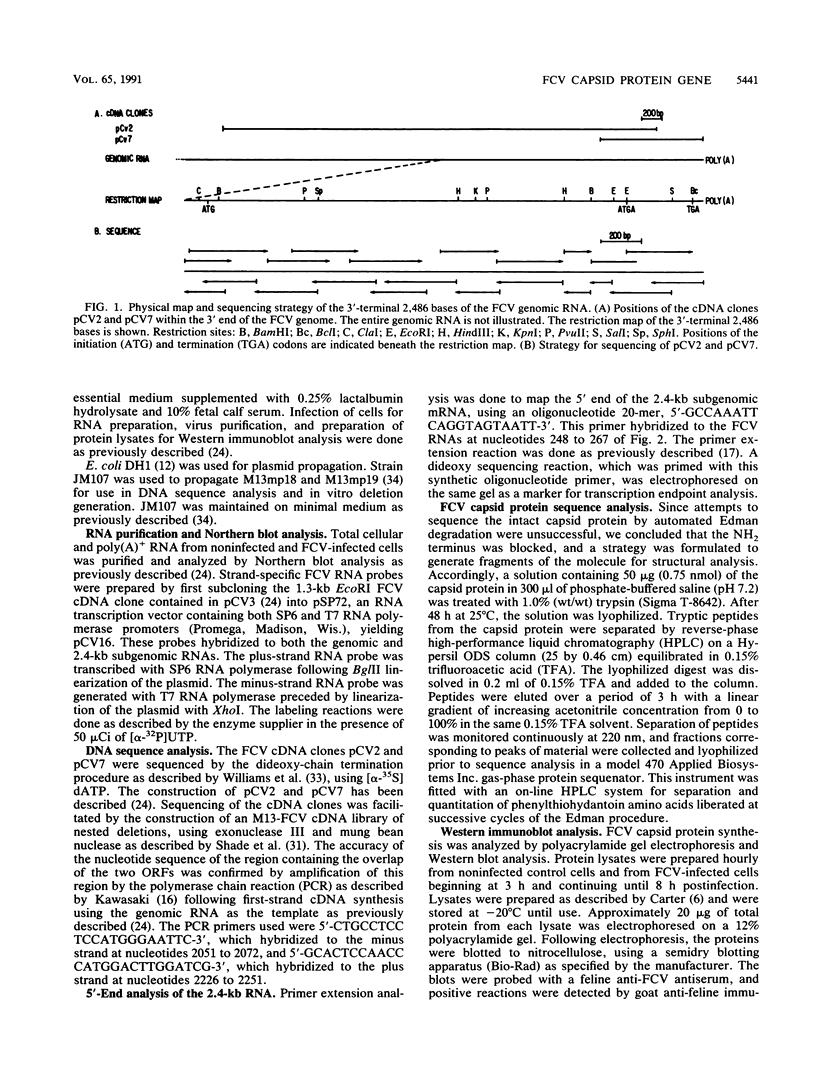
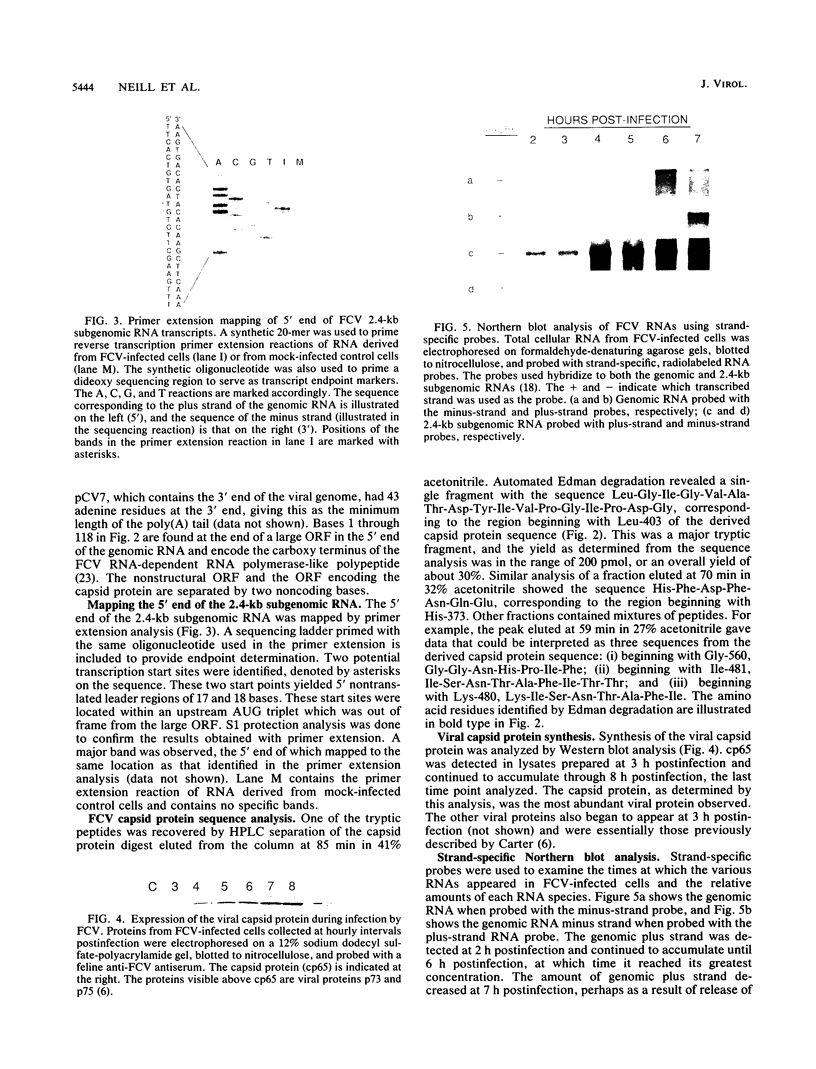
The sequence of the 3'-terminal 2,486 bases of the feline calicivirus (FCV) genome was determined. This region of the FCV genome, from which the 2.4-kb subgenomic RNA is derived, contained two open reading frames. The larger open reading frame, found in the 5' end of the subgenomic mRNA, contained 2,004 bases encoding a polypeptide of 73,467 Da. The smaller open reading frame, encoded in the 3' end of the mRNA, was composed of 318 bases, encoding a polypeptide of 12,185 Da. The AUG initiation codon of the second open reading frame overlapped the UGA termination codon of the first, with the sequence AUGA. The nucleotide sequence of the region containing this overlap resembles the -1 frameshift sequences of the retroviruses. The 5' end of the 2.4-kb subgenomic RNA was mapped by primer extension analysis. There were two apparent transcription initiation points, both of which were 5' to the AUG initiation codon of the large open reading frame. Transcription from these sites yielded RNA transcripts with 5' nontranslated leader regions of 17 and 18 bases. The total length of the 2.4-kb subgenomic RNA was 2,375 bases (from the 5'-most start site) excluding the poly(A) tail. Edman degradation of the purified capsid protein of FCV showed that the capsid protein was encoded by the large open reading frame. Western immunoblot analysis of FCV-infected cells using a feline anti-FCV antiserum demonstrated that translation of the capsid protein was detectable at 3 h postinfection and continued to accumulate until 8 h postinfection, the last time examined.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachrach H. L., Hess W. R. Animal picornaviruses with a single major species of capsid protein. Biochem Biophys Res Commun. 1973 Nov 1;55(1):141–149. doi: 10.1016/s0006-291x(73)80070-1. [DOI] [PubMed] [Google Scholar]
- Black D. N., Burroughs J. N., Harris T. J., Brown F. The structure and replication of calicivirus RNA. Nature. 1978 Aug 10;274(5671):614–615. doi: 10.1038/274614a0. [DOI] [PubMed] [Google Scholar]
- Brierley I., Digard P., Inglis S. C. Characterization of an efficient coronavirus ribosomal frameshifting signal: requirement for an RNA pseudoknot. Cell. 1989 May 19;57(4):537–547. doi: 10.1016/0092-8674(89)90124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burroughs J. N., Brown F. Presence of a covalently linked protein on calicivirus RNA. J Gen Virol. 1978 Nov;41(2):443–446. doi: 10.1099/0022-1317-41-2-443. [DOI] [PubMed] [Google Scholar]
- Burroughs J. N., Doel T. R., Smale C. J., Brown F. A model for vesicular exanthema virus, the prototype of the calicivirus group. J Gen Virol. 1978 Jul;40(1):161–174. doi: 10.1099/0022-1317-40-1-161. [DOI] [PubMed] [Google Scholar]
- Carter M. J. Feline calicivirus protein synthesis investigated by western blotting. Arch Virol. 1989;108(1-2):69–79. doi: 10.1007/BF01313744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. J., Routledge E. G., Toms G. L. Monoclonal antibodies to feline calicivirus. J Gen Virol. 1989 Aug;70(Pt 8):2197–2200. doi: 10.1099/0022-1317-70-8-2197. [DOI] [PubMed] [Google Scholar]
- Dasgupta R., Shih D. S., Saris C., Kaesberg P. Nucleotide sequence of a viral RNA fragment that binds to eukaryotic ribosomes. Nature. 1975 Aug 21;256(5519):624–628. doi: 10.1038/256624a0. [DOI] [PubMed] [Google Scholar]
- Ehresmann D. W., Schaffer F. L. Calicivirus intracellular RNA: fractionation of 18-22 s RNA and lack of typical 5'-methylated caps on 36 S and 22 S San Miguel sea lion virus RNAs. Virology. 1979 May;95(1):251–255. doi: 10.1016/0042-6822(79)90426-4. [DOI] [PubMed] [Google Scholar]
- Ehresmann D. W., Schaffer F. L. RNA synthesized in calicivirus-infected cells is atypical of picornaviruses. J Virol. 1977 May;22(2):572–576. doi: 10.1128/jvi.22.2.572-576.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fretz M., Schaffer F. L. Calicivirus proteins in infected cells: evidence for a capsid polypeptide precursor. Virology. 1978 Aug;89(1):318–321. doi: 10.1016/0042-6822(78)90065-x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hizi A., Henderson L. E., Copeland T. D., Sowder R. C., Hixson C. V., Oroszlan S. Characterization of mouse mammary tumor virus gag-pro gene products and the ribosomal frameshift site by protein sequencing. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7041–7045. doi: 10.1073/pnas.84.20.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Madhani H. D., Masiarz F. R., Varmus H. E. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell. 1988 Nov 4;55(3):447–458. doi: 10.1016/0092-8674(88)90031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Townsley K., Varmus H. E., Majors J. Two efficient ribosomal frameshifting events are required for synthesis of mouse mammary tumor virus gag-related polyproteins. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4298–4302. doi: 10.1073/pnas.84.12.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komolafe O. O., Jarrett O., Neil J. C. Feline calicivirus induced polypeptides. Microbios. 1980;27(109 110):185–192. [PubMed] [Google Scholar]
- Koonin E. V., Boyko V. P., Dolja V. V. Small cysteine-rich proteins of different groups of plant RNA viruses are related to different families of nucleic acid-binding proteins. Virology. 1991 Mar;181(1):395–398. doi: 10.1016/0042-6822(91)90512-a. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill J. D., Mengeling W. L. Further characterization of the virus-specific RNAs in feline calicivirus infected cells. Virus Res. 1988 Aug;11(1):59–72. doi: 10.1016/0168-1702(88)90067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill J. D. Nucleotide sequence of a region of the feline calicivirus genome which encodes picornavirus-like RNA-dependent RNA polymerase, cysteine protease and 2C polypeptides. Virus Res. 1990 Nov;17(3):145–160. doi: 10.1016/0168-1702(90)90061-f. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Rowlands D. J., Brown F. A physico-chemical sub-grouping of the mammalian picornaviruses. J Gen Virol. 1973 Feb;18(2):171–180. doi: 10.1099/0022-1317-18-2-171. [DOI] [PubMed] [Google Scholar]
- Oglesby A. S., Schaffer F. L., Madin S. H. Biochemical and biophysical properties of vesicular exanthema of swine virus. Virology. 1971 May;44(2):329–341. doi: 10.1016/0042-6822(71)90264-9. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Complete intergenic and flanking gene sequences from the genome of vesicular stomatitis virus. Cell. 1980 Feb;19(2):415–421. doi: 10.1016/0092-8674(80)90515-2. [DOI] [PubMed] [Google Scholar]
- Schaffer F. L., Ehresmann D. W., Fretz M. K., Soergel M. I. A protein, VPg, covalently linked to 36S calicivirus RNA. J Gen Virol. 1980 Mar;47(1):215–220. doi: 10.1099/0022-1317-47-1-215. [DOI] [PubMed] [Google Scholar]
- Schaffer F. L., Soergel M. E. Single major polypeptide of a calicivirus: characterization by polyacrylamide gel electrophoresis and stabilization of virions by cross-linking with dimethyl suberimidate. J Virol. 1976 Sep;19(3):925–931. doi: 10.1128/jvi.19.3.925-931.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shade R. O., Blundell M. C., Cotmore S. F., Tattersall P., Astell C. R. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol. 1986 Jun;58(3):921–936. doi: 10.1128/jvi.58.3.921-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soergel M. E., Akers T. G., Schaffer F. L., Noma A. T. Amino acid composition of three immunological types of a calicivirus, San Miguel sea lion virus. Virology. 1976 Jul 15;72(2):527–529. doi: 10.1016/0042-6822(76)90183-5. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]