Abstract
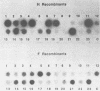
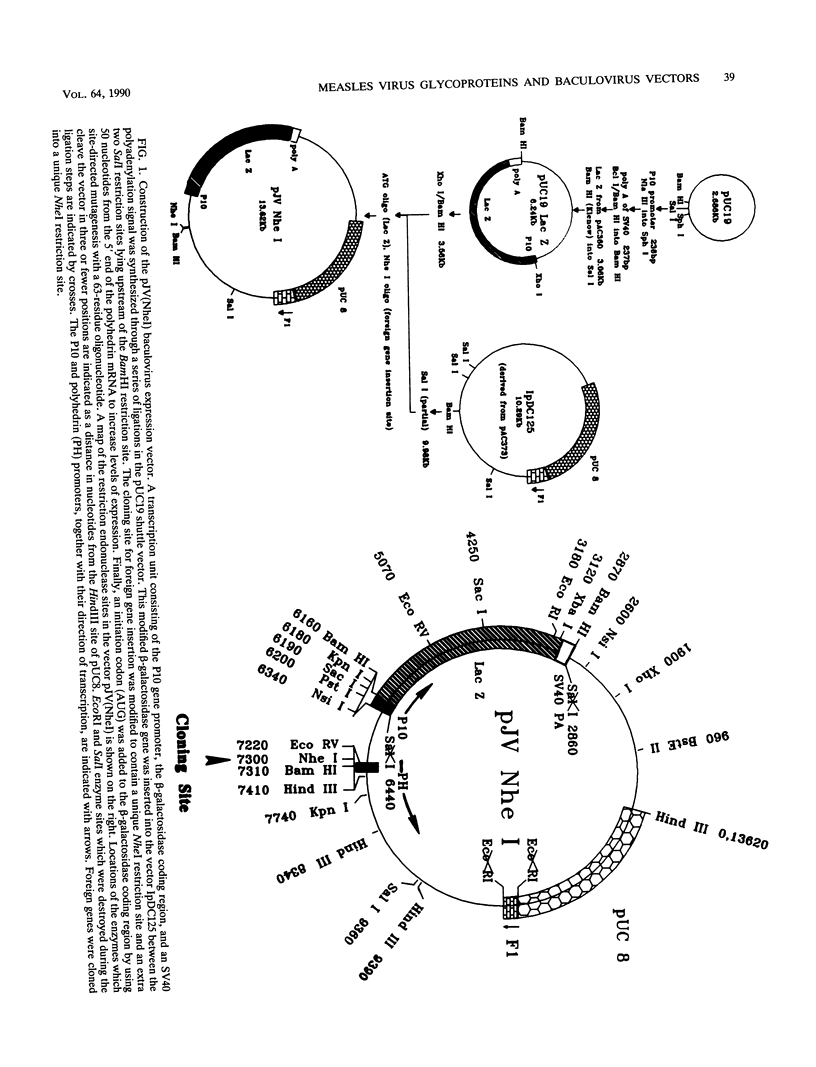
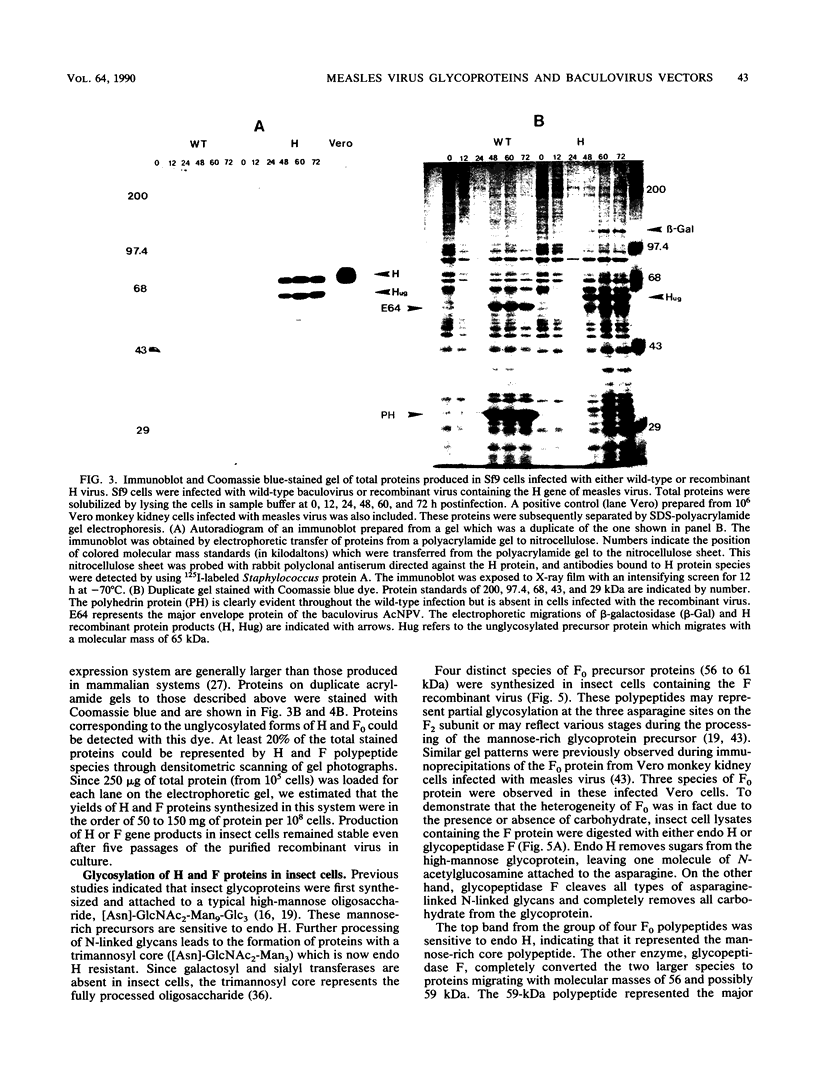
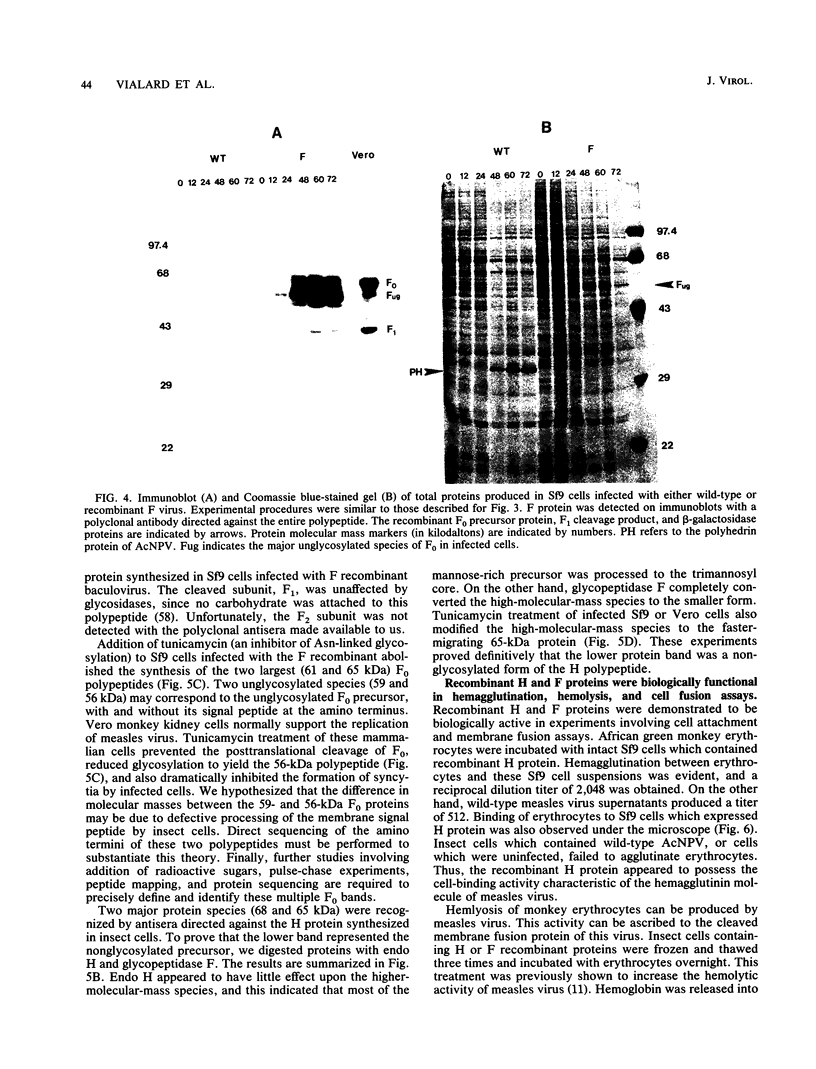
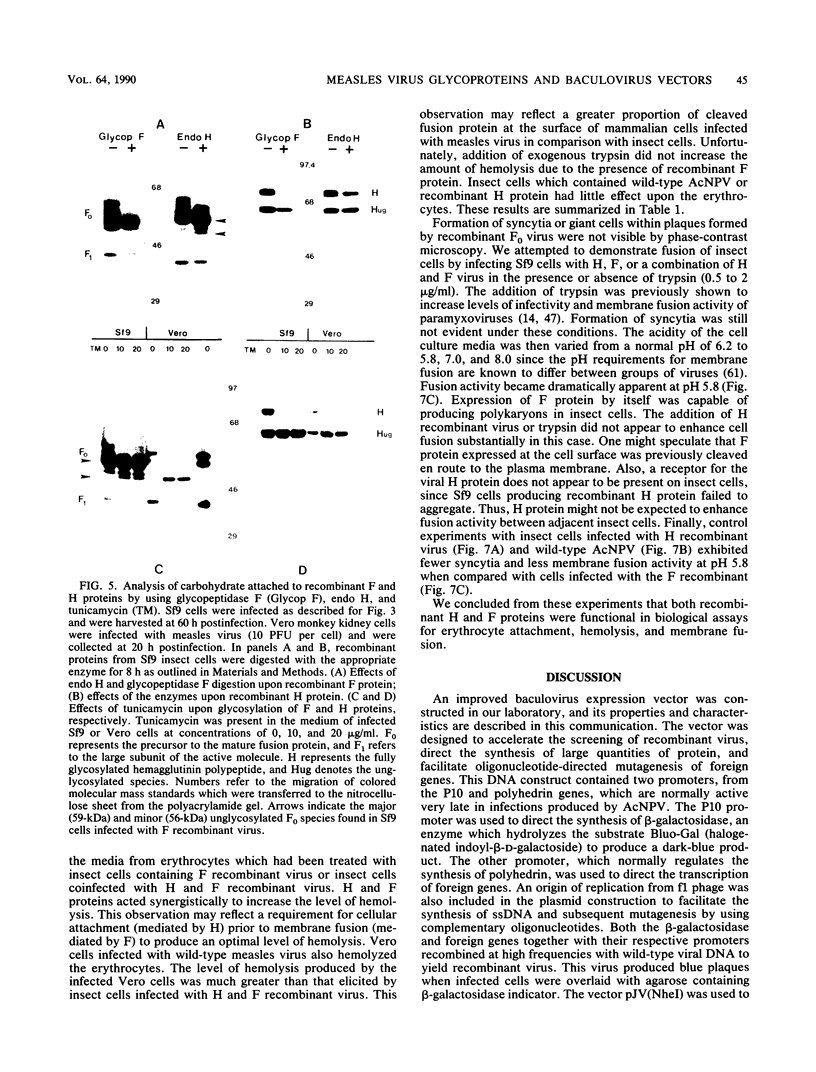
An improved baculovirus expression vector was developed to expedite screening and facilitate oligonucleotide-directed mutagenesis. This vector contained twin promoters derived from the P10 and polyhedrin genes of Autographica californica nuclear polyhedrosis virus. The P10 promoter directed the synthesis of beta-galactosidase, whereas the polyhedrin promoter controlled the synthesis of foreign gene products. These two genes recombined with wild-type virus genome to yield recombinants which were polyhedrin negative, produced the foreign gene product, and formed blue plaques when beta-galactosidase indicator was present in the agarose overlay. An origin of replication derived from M13 or f1 bacteriophage was also included in the plasmid to permit the synthesis of single-stranded DNA. This template DNA was used to introduce or delete sequences through the process of site-specific mutagenesis. The measles virus virion possesses a membrane envelope which contains two glycoproteins: the hemagglutinin (H) and membrane fusion (F) proteins. The H polypeptide has receptor-binding and hemagglutinating activity, whereas the F protein mediates virus penetration of the host cell, formation of syncytia, and hemolysis of erythrocytes. Genes for these two glycoproteins were inserted into the NheI cloning site of the modified expression vector described above. The vector and purified wild-type viral DNA were introduced into Sf9 insect cells by calcium phosphate precipitation. A mixture of wild-type and recombinant virus was generated and used to infect Sf9 cells, which were subsequently overlaid with agarose. After 3 days, 0.1 to 1% of the plaques became blue in the presence of beta-galactosidase indicator. At least 70% of these blue viral colonies contained the foreign gene of interest as determined by dot blot analysis. Recombinant virus was separated from contaminating wild-type virus through several rounds of plaque purification. Insect cells were then infected with the purified recombinants, and synthesis of H and F proteins were verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by immunoblot detection and Coomassie blue staining. Glycosylation of the proteins appeared to be impaired somewhat, and the precursor to the F protein was not completely cleaved by the proteases present in insect host cells. On the other hand, both proteins appeared to be active in hemagglutination, hemolysis, and cell fusion assays. Levels of synthesis were in the order of 50 to 150 mg of protein per 10(8) cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkhatib G., Briedis D. J. High-level eucaryotic in vivo expression of biologically active measles virus hemagglutinin by using an adenovirus type 5 helper-free vector system. J Virol. 1988 Aug;62(8):2718–2727. doi: 10.1128/jvi.62.8.2718-2727.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib G., Briedis D. J. The predicted primary structure of the measles virus hemagglutinin. Virology. 1986 Apr 30;150(2):479–490. doi: 10.1016/0042-6822(86)90312-0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carbonell L. F., Klowden M. J., Miller L. K. Baculovirus-mediated expression of bacterial genes in dipteran and mammalian cells. J Virol. 1985 Oct;56(1):153–160. doi: 10.1128/jvi.56.1.153-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R. S., Downie J. C., Hay A. J., Knossow M., Skehel J. J., Wang M. L., Wiley D. C. Fusion mutants of the influenza virus hemagglutinin glycoprotein. Cell. 1985 Feb;40(2):431–439. doi: 10.1016/0092-8674(85)90157-6. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Helenius A., White J. Membrane fusion activity of the influenza virus hemagglutinin. The low pH-induced conformational change. J Biol Chem. 1985 Mar 10;260(5):2973–2981. [PubMed] [Google Scholar]
- Emery V. C., Bishop D. H. The development of multiple expression vectors for high level synthesis of eukaryotic proteins: expression of LCMV-N and AcNPV polyhedrin protein by a recombinant baculovirus. Protein Eng. 1987 Aug-Sep;1(4):359–366. doi: 10.1093/protein/1.4.359. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Doms R. W., York D., White J. Studies on the mechanism of membrane fusion: site-specific mutagenesis of the hemagglutinin of influenza virus. J Cell Biol. 1986 Jan;102(1):11–23. doi: 10.1083/jcb.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P., Robbins P. W. Regulation of asparagine-linked oligosaccharide processing. Oligosaccharide processing in Aedes albopictus mosquito cells. J Biol Chem. 1984 Feb 25;259(4):2375–2382. [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Enhancement of membrane-fusing activity of sendai virus by exposure of the virus to basic pH is correlated with a conformational change in the fusion protein. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5862–5866. doi: 10.1073/pnas.79.19.5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. I., Kosowski S. G., Schaaf K. F. Expression of envelope glycoproteins of human immunodeficiency virus by an insect virus vector. J Virol. 1987 Nov;61(11):3617–3620. doi: 10.1128/jvi.61.11.3617-3620.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis D. L., Summers M. D. Glycosylation and secretion of human tissue plasminogen activator in recombinant baculovirus-infected insect cells. Mol Cell Biol. 1989 Jan;9(1):214–223. doi: 10.1128/mcb.9.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. W., Attia J., Richardson C. D., Roder J. C., Dunn R. J. Synthesis of soluble myelin-associated glycoprotein in insect and mammalian cells. Gene. 1989 Apr 30;77(2):287–296. doi: 10.1016/0378-1119(89)90076-0. [DOI] [PubMed] [Google Scholar]
- Knebel D., Lübbert H., Doerfler W. The promoter of the late p10 gene in the insect nuclear polyhedrosis virus Autographa californica: activation by viral gene products and sensitivity to DNA methylation. EMBO J. 1985 May;4(5):1301–1306. doi: 10.1002/j.1460-2075.1985.tb03776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kuroda K., Hauser C., Rott R., Klenk H. D., Doerfler W. Expression of the influenza virus haemagglutinin in insect cells by a baculovirus vector. EMBO J. 1986 Jun;5(6):1359–1365. doi: 10.1002/j.1460-2075.1986.tb04367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzio J., Rohel D. Z., Curry C. J., Krebs A., Carstens E. B., Faulkner P. Nucleotide sequence of the p10 polypeptide gene of Autographa californica nuclear polyhedrosis virus. Virology. 1984 Dec;139(2):414–418. doi: 10.1016/0042-6822(84)90388-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Feinberg M. B., Reyes G. R., Rabin L., Banapour B., Chakrabarti S., Moss B., Wong-Staal F., Steimer K. S., Engleman E. G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986 Oct 23;323(6090):725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. High level expression of nonfused foreign genes with Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1989 May;170(1):31–39. doi: 10.1016/0042-6822(89)90348-6. [DOI] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. Signals important for high-level expression of foreign genes in Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1988 Nov;167(1):56–71. doi: 10.1016/0042-6822(88)90054-2. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Maeda S., Kawai T., Obinata M., Fujiwara H., Horiuchi T., Saeki Y., Sato Y., Furusawa M. Production of human alpha-interferon in silkworm using a baculovirus vector. Nature. 1985 Jun 13;315(6020):592–594. doi: 10.1038/315592a0. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Possee R. D., Overton H. A., Bishop D. H. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J Gen Virol. 1987 May;68(Pt 5):1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Miller L. K. Baculoviruses as gene expression vectors. Annu Rev Microbiol. 1988;42:177–199. doi: 10.1146/annurev.mi.42.100188.001141. [DOI] [PubMed] [Google Scholar]
- Morrison T., Ward L. J., Semerjian A. Intracellular processing of the Newcastle disease virus fusion glycoprotein. J Virol. 1985 Mar;53(3):851–857. doi: 10.1128/jvi.53.3.851-857.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. G., Hiebert S. W., Lamb R. A. Expression at the cell surface of biologically active fusion and hemagglutinin/neuraminidase proteins of the paramyxovirus simian virus 5 from cloned cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7520–7524. doi: 10.1073/pnas.82.22.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. G., Lamb R. A., Moss B., Murphy B. R. Comparison of the relative roles of the F and HN surface glycoproteins of the paramyxovirus simian virus 5 in inducing protective immunity. J Virol. 1987 Jun;61(6):1972–1977. doi: 10.1128/jvi.61.6.1972-1977.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennock G. D., Shoemaker C., Miller L. K. Strong and regulated expression of Escherichia coli beta-galactosidase in insect cells with a baculovirus vector. Mol Cell Biol. 1984 Mar;4(3):399–406. doi: 10.1128/mcb.4.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possee R. D. Cell-surface expression of influenza virus haemagglutinin in insect cells using a baculovirus vector. Virus Res. 1986 Jul;5(1):43–59. doi: 10.1016/0168-1702(86)90064-x. [DOI] [PubMed] [Google Scholar]
- Possee R. D., Howard S. C. Analysis of the polyhedrin gene promoter of the Autographa californica nuclear polyhedrosis virus. Nucleic Acids Res. 1987 Dec 23;15(24):10233–10248. doi: 10.1093/nar/15.24.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. D., Berkovich A., Rozenblatt S., Bellini W. J. Use of antibodies directed against synthetic peptides for identifying cDNA clones, establishing reading frames, and deducing the gene order of measles virus. J Virol. 1985 Apr;54(1):186–193. doi: 10.1128/jvi.54.1.186-193.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C., Hull D., Greer P., Hasel K., Berkovich A., Englund G., Bellini W., Rima B., Lazzarini R. The nucleotide sequence of the mRNA encoding the fusion protein of measles virus (Edmonston strain): a comparison of fusion proteins from several different paramyxoviruses. Virology. 1986 Dec;155(2):508–523. doi: 10.1016/0042-6822(86)90212-6. [DOI] [PubMed] [Google Scholar]
- Ronin C., Papandreou M. J., Canonne C., Weintraub B. D. Carbohydrate chains of human thyrotropin are differentially susceptible to endoglycosidase removal on combined and free polypeptide subunits. Biochemistry. 1987 Sep 8;26(18):5848–5853. doi: 10.1021/bi00392a040. [DOI] [PubMed] [Google Scholar]
- Russel M., Kidd S., Kelley M. R. An improved filamentous helper phage for generating single-stranded plasmid DNA. Gene. 1986;45(3):333–338. doi: 10.1016/0378-1119(86)90032-6. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Protease activation mutants of sendai virus. Activation of biological properties by specific proteases. Virology. 1976 Jan;69(1):265–277. doi: 10.1016/0042-6822(76)90213-0. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virology. 1977 Jul 1;80(1):54–66. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Ju G., Ericson B. L., Moschera J., Lahm H. W., Chizzonite R., Summers M. D. Modification and secretion of human interleukin 2 produced in insect cells by a baculovirus expression vector. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8404–8408. doi: 10.1073/pnas.82.24.8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D., Fraser M. J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol Cell Biol. 1983 Dec;3(12):2156–2165. doi: 10.1128/mcb.3.12.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Vlak J. M., Summers M. D. Physical Analysis of Autographa californica Nuclear Polyhedrosis Virus Transcripts for Polyhedrin and 10,000-Molecular-Weight Protein. J Virol. 1983 Jan;45(1):215–225. doi: 10.1128/jvi.45.1.215-225.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Murphy B. R., Prince G. A., Olmsted R. A., Collins P. L. Expression of the F and HN glycoproteins of human parainfluenza virus type 3 by recombinant vaccinia viruses: contributions of the individual proteins to host immunity. J Virol. 1987 Nov;61(11):3416–3423. doi: 10.1128/jvi.61.11.3416-3423.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao H., Liu G. Q., Ruan L., Chu C. M. Construction and application of plasmids containing bidirectional promoters of vaccinia virus. J Virol. 1988 Dec;62(12):4832–4834. doi: 10.1128/jvi.62.12.4832-4834.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsanyi T. M., Utter G., Norrby E. Purification, morphology and antigenic characterization of measles virus envelope components. J Gen Virol. 1984 Feb;65(Pt 2):355–366. doi: 10.1099/0022-1317-65-2-355. [DOI] [PubMed] [Google Scholar]
- Volkman L. E. The 64K envelope protein of budded Autographa californica nuclear polyhedrosis virus. Curr Top Microbiol Immunol. 1986;131:103–118. doi: 10.1007/978-3-642-71589-1_6. [DOI] [PubMed] [Google Scholar]
- Wertz G. W., Stott E. J., Young K. K., Anderson K., Ball L. A. Expression of the fusion protein of human respiratory syncytial virus from recombinant vaccinia virus vectors and protection of vaccinated mice. J Virol. 1987 Feb;61(2):293–301. doi: 10.1128/jvi.61.2.293-301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Murphy B. R., Collins P. L., Lebacq-Verheyden A. M., Battey J. F. Expression of biologically active and antigenically authentic parainfluenza type 3 virus hemagglutinin-neuraminidase glycoprotein by a recombinant baculovirus. Virology. 1987 Oct;160(2):465–472. doi: 10.1016/0042-6822(87)90018-3. [DOI] [PubMed] [Google Scholar]