Abstract
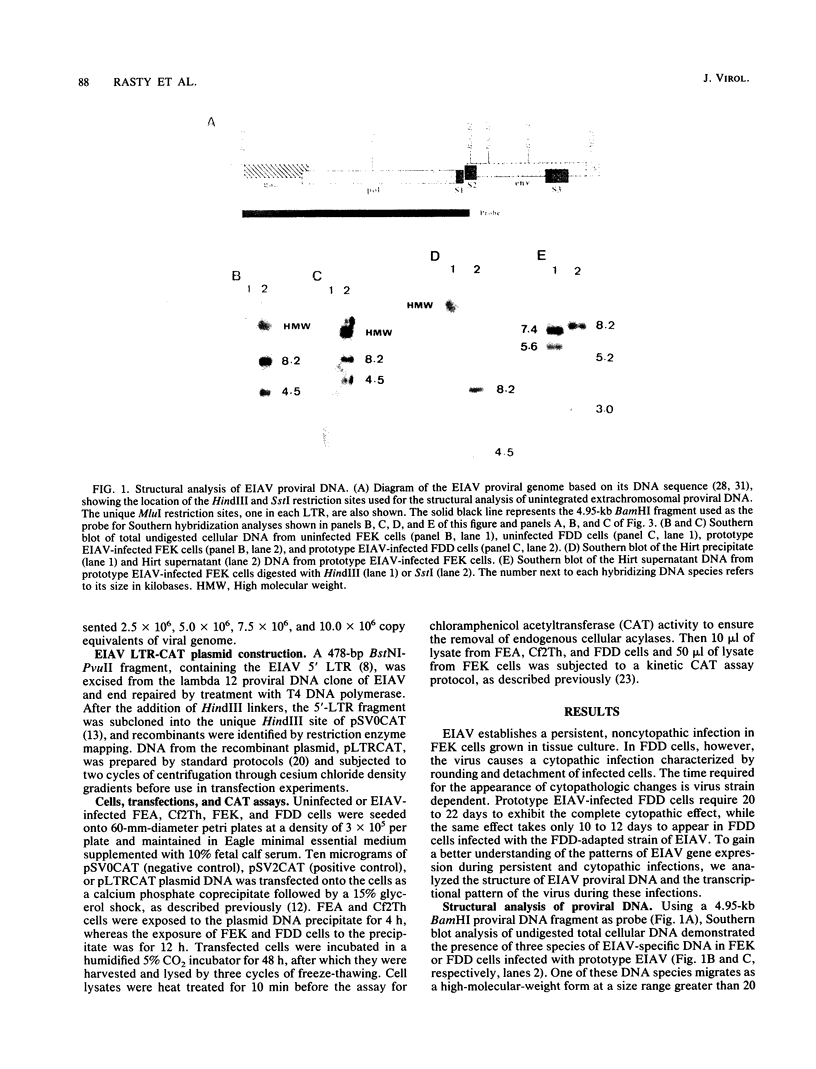
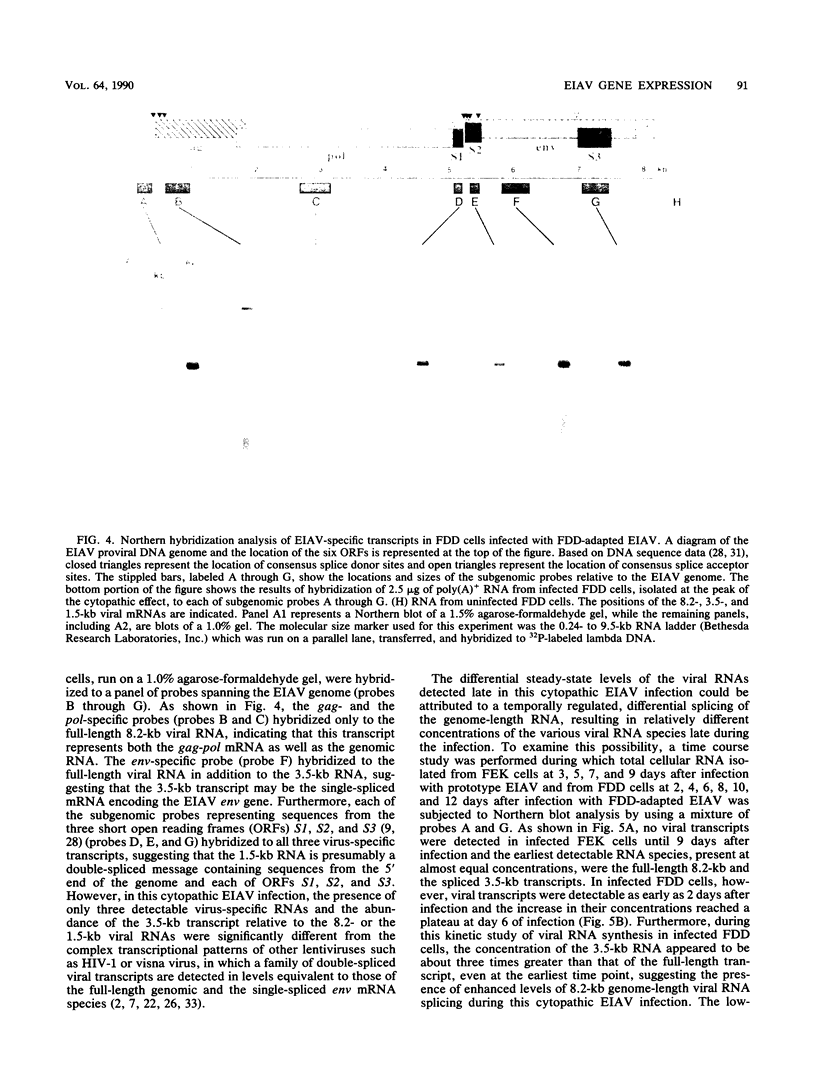
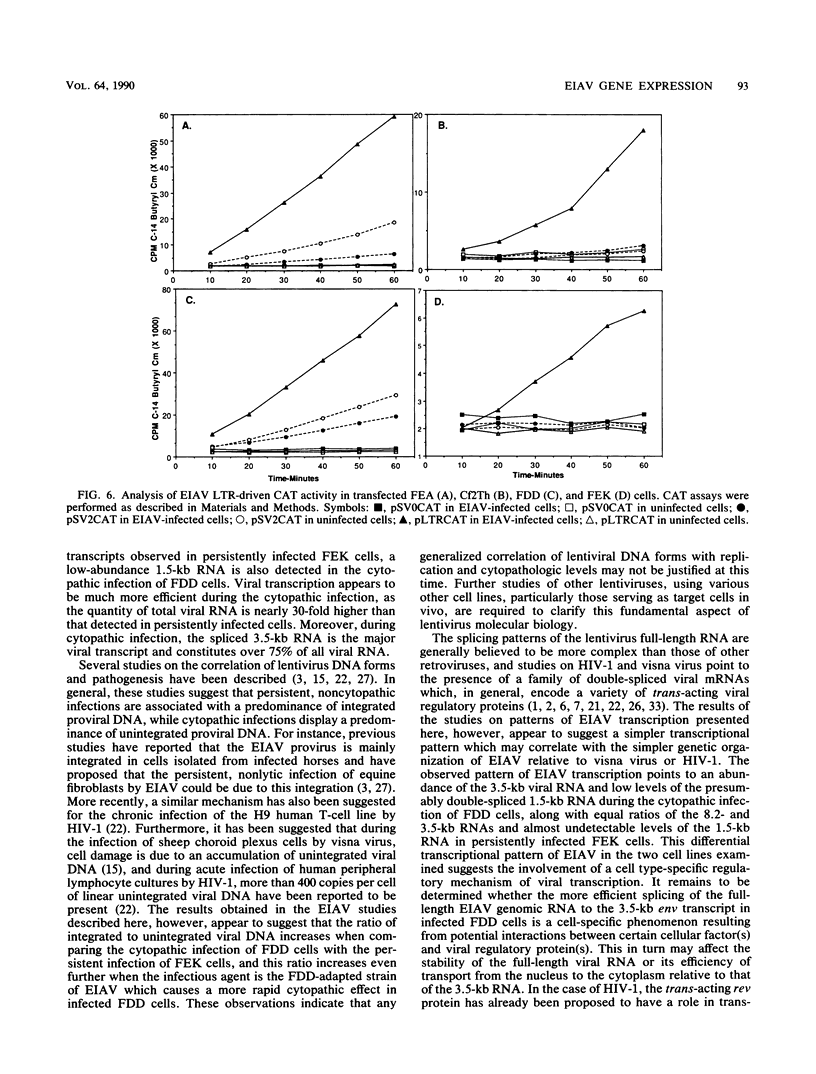
The structure and integration patterns of equine infectious anemia virus (EIAV) proviral DNA and the patterns of viral transcription were examined in persistent and cytopathic infections of cultured cells. The results of Southern blot analyses indicated that, in persistently infected cells, about 30% of the EIAV provirus exists as randomly integrated DNA, while the remaining 70% is equally divided between unintegrated linear and closed circular forms. The cytopathic infection, in contrast, is characterized by levels of integrated provirus ranging from 65 to more than 90% of the total proviral DNA, depending on the extent of cytopathology exhibited by the virus strain employed. In both persistent and cytopathic infections, extensive Northern (RNA) blot analyses have revealed the presence of two major virus-specific transcripts, an 8.2-kilobase (kb) full-length genomic mRNA and a 3.5-kb single-spliced mRNA. A low-abundance 1.5-kb mRNA, presumably formed by a double-splicing event of the full-length RNA, was also detected in the cytopathic EIAV infection. The two major viral transcripts are present in approximately equal quantities in persistently infected cells, while the cytopathic infection reveals nearly a 30-fold higher level of viral transcripts in which the 3.5-kb species constitutes over 75% of the total viral mRNA. The relatively high proportion of proviral DNA integration and the simple pattern of viral transcription observed during EIAV infections appeared to be different from the generally observed patterns of predominantly unintegrated proviral DNA and multi-spliced viral mRNAs in cells infected with other lentiviruses such as visna virus or human immunodeficiency virus type 1. Moreover, the data suggested that the cytopathology of EIAV may be correlated in part with the degree of proviral DNA integration and levels of viral mRNA in infected cells, particularly that of the spliced 3.5-kb mRNA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Gallo R. C. Three novel genes of human T-lymphotropic virus type III: immune reactivity of their products with sera from acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2209–2213. doi: 10.1073/pnas.83.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Cheevers W. P., Watson S. G., Klevjer-Anderson P. Persistent infection by equine infectious anemia virus: asymmetry of nucleotide sequence reiteration in the integrated provirus of persistently infected cells. Virology. 1982 Apr 15;118(1):246–253. doi: 10.1016/0042-6822(82)90340-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clements J. E., Narayan O., Griffin D. E., Johnson R. T. The synthesis and structure of visna virus DNA. Virology. 1979 Mar;93(2):377–386. doi: 10.1016/0042-6822(79)90242-3. [DOI] [PubMed] [Google Scholar]
- Davis J. L., Clements J. E. Characterization of a cDNA clone encoding the visna virus transactivating protein. Proc Natl Acad Sci U S A. 1989 Jan;86(2):414–418. doi: 10.1073/pnas.86.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. L., Molineaux S., Clements J. E. Visna virus exhibits a complex transcriptional pattern: one aspect of gene expression shared with the acquired immunodeficiency syndrome retrovirus. J Virol. 1987 May;61(5):1325–1331. doi: 10.1128/jvi.61.5.1325-1331.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derse D., Dorn P. L., Levy L., Stephens R. M., Rice N. R., Casey J. W. Characterization of equine infectious anemia virus long terminal repeat. J Virol. 1987 Mar;61(3):743–747. doi: 10.1128/jvi.61.3.743-747.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn P. L., Derse D. cis- and trans-acting regulation of gene expression of equine infectious anemia virus. J Virol. 1988 Sep;62(9):3522–3526. doi: 10.1128/jvi.62.9.3522-3526.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Hadzopoulou-Cladaras M., Cladaras C., Copeland T., Pavlakis G. N. rev protein of human immunodeficiency virus type 1 affects the stability and transport of the viral mRNA. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1495–1499. doi: 10.1073/pnas.86.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordis C. M., Howard B. H. Use of the CAT reporter gene for optimization of gene transfer into eukaryotic cells. Methods Enzymol. 1987;151:382–397. doi: 10.1016/s0076-6879(87)51030-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarskjöld M. L., Heimer J., Hammarskjöld B., Sangwan I., Albert L., Rekosh D. Regulation of human immunodeficiency virus env expression by the rev gene product. J Virol. 1989 May;63(5):1959–1966. doi: 10.1128/jvi.63.5.1959-1966.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. D., Blum H., Scott J., Traynor B., Ventura P., Haase A. Slow virus visna: reproduction in vitro of virus from extrachromosomal DNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7212–7215. doi: 10.1073/pnas.81.22.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Le S. Y., Maizel J. V., Cullen B. R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989 Mar 16;338(6212):254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- Malmquist W. A., Barnett D., Becvar C. S. Production of equine infectious anemia antigen in a persistently infected cell line. Arch Gesamte Virusforsch. 1973;42(4):361–370. doi: 10.1007/BF01250717. [DOI] [PubMed] [Google Scholar]
- Mazarin V., Gourdou I., Quérat G., Sauze N., Vigne R. Genetic structure and function of an early transcript of visna virus. J Virol. 1988 Dec;62(12):4813–4818. doi: 10.1128/jvi.62.12.4813-4818.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Orrego A., Issel C. J., Montelaro R. C., Adams W. V., Jr Virulence and in vitro growth of a cell-adapted strain of equine infectious anemia virus after serial passage in ponies. Am J Vet Res. 1982 Sep;43(9):1556–1560. [PubMed] [Google Scholar]
- Payne S. L., Fang F. D., Liu C. P., Dhruva B. R., Rwambo P., Issel C. J., Montelaro R. C. Antigenic variation and lentivirus persistence: variations in envelope gene sequences during EIAV infection resemble changes reported for sequential isolates of HIV. Virology. 1987 Dec;161(2):321–331. doi: 10.1016/0042-6822(87)90124-3. [DOI] [PubMed] [Google Scholar]
- Rabson A. B., Daugherty D. F., Venkatesan S., Boulukos K. E., Benn S. I., Folks T. M., Feorino P., Martin M. A. Transcription of novel open reading frames of AIDS retrovirus during infection of lymphocytes. Science. 1985 Sep 27;229(4720):1388–1390. doi: 10.1126/science.2994220. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Simek S., Ryder O. A., Coggins L. Detection of proviral DNA in horse cells infected with equine infectious anemia virus. J Virol. 1978 Jun;26(3):577–583. doi: 10.1128/jvi.26.3.577-583.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushlow K., Olsen K., Stiegler G., Payne S. L., Montelaro R. C., Issel C. J. Lentivirus genomic organization: the complete nucleotide sequence of the env gene region of equine infectious anemia virus. Virology. 1986 Dec;155(2):309–321. doi: 10.1016/0042-6822(86)90195-9. [DOI] [PubMed] [Google Scholar]
- Sherman L., Gazit A., Yaniv A., Kawakami T., Dahlberg J. E., Tronick S. R. Localization of sequences responsible for trans-activation of the equine infectious anemia virus long terminal repeat. J Virol. 1988 Jan;62(1):120–126. doi: 10.1128/jvi.62.1.120-126.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Stephens R. M., Casey J. W., Rice N. R. Equine infectious anemia virus gag and pol genes: relatedness to visna and AIDS virus. Science. 1986 Feb 7;231(4738):589–594. doi: 10.1126/science.3003905. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Vigne R., Barban V., Quérat G., Mazarin V., Gourdou I., Sauze N. Transcription of visna virus during its lytic cycle: evidence for a sequential early and late gene expression. Virology. 1987 Nov;161(1):218–227. doi: 10.1016/0042-6822(87)90188-7. [DOI] [PubMed] [Google Scholar]