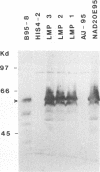
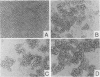
Abstract
Expression of the Epstein-Barr virus BNLF1 gene (LMP1) or treatment with 4B-phorbol-12,13-dibutyrate in the murine pro-B-cell line A/J-95 leads to a five- to sevenfold enhancement of homotypic adhesion without significant changes in adhesion molecule expression. Antibody to CD11a inhibits aggregation, indicating that CD11a/CD18-mediated adhesion is a common target for both agents.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Ben-Bassat H., Goldblum N., Mitrani S., Goldblum T., Yoffey J. M., Cohen M. M., Bentwich Z., Ramot B., Klein E., Klein G. Establishment in continuous culture of a new type of lymphocyte from a "Burkitt like" malignant lymphoma (line D.G.-75). Int J Cancer. 1977 Jan;19(1):27–33. doi: 10.1002/ijc.2910190105. [DOI] [PubMed] [Google Scholar]
- Birkenbach M., Liebowitz D., Wang F., Sample J., Kieff E. Epstein-Barr virus latent infection membrane protein increases vimentin expression in human B-cell lines. J Virol. 1989 Sep;63(9):4079–4084. doi: 10.1128/jvi.63.9.4079-4084.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J. Spontaneous in vitro occurrence and long-term culture of murine B lymphoblast cell lines. J Immunol. 1983 May;130(5):2113–2116. [PubMed] [Google Scholar]
- Chatila T. A., Geha R. S., Arnaout M. A. Constitutive and stimulus-induced phosphorylation of CD11/CD18 leukocyte adhesion molecules. J Cell Biol. 1989 Dec;109(6 Pt 2):3435–3444. doi: 10.1083/jcb.109.6.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri Y., Braun J., Baltimore D. Elevated myc expression and c-myc amplification in spontaneously occurring B lymphoid cell lines. J Exp Med. 1987 Apr 1;165(4):1188–1194. doi: 10.1084/jem.165.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbere-Garapin F., Chousterman S., Horodniceanu F., Kourilsky P., Garapin A. C. Cloning of the active thymidine kinase gene of herpes simplex virus type 1 in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3755–3759. doi: 10.1073/pnas.76.8.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo L., Trivedi P., Wang F., Winberg G., Klein G., Masucci M. G. Expression of the Epstein-Barr virus (EBV)-encoded membrane antigen (LMP) increases the stimulatory capacity of EBV-negative B lymphoma lines in allogeneic mixed lymphocyte cultures. Eur J Immunol. 1990 Oct;20(10):2293–2299. doi: 10.1002/eji.1830201019. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Ernberg I., Kallin B., Dillner J., Falk K., Ehlin-Henriksson B., Hammarskjöld M. L., Klein G. Lymphoblastoid cell lines and Burkitt-lymphoma-derived cell lines differ in the expression of a second Epstein-Barr virus encoded nuclear antigen. Int J Cancer. 1986 Nov 15;38(5):729–737. doi: 10.1002/ijc.2910380517. [DOI] [PubMed] [Google Scholar]
- Franz T., Hilberg F., Seliger B., Stocking C., Ostertag W. Retroviral mutants efficiently expressed in embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3292–3296. doi: 10.1073/pnas.83.10.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E., Kolbe M., Noonan K., Kucherlapati R. Construction of a mammalian transducing vector from the genome of Moloney murine leukemia virus. J Virol. 1982 Dec;44(3):845–851. doi: 10.1128/jvi.44.3.845-851.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman S. C., Mulligan R. C. Two dominant-acting selectable markers for gene transfer studies in mammalian cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8047–8051. doi: 10.1073/pnas.85.21.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs M. L., Xu H., Stacker S. A., Springer T. A. Regulation of adhesion of ICAM-1 by the cytoplasmic domain of LFA-1 integrin beta subunit. Science. 1991 Mar 29;251(5001):1611–1613. doi: 10.1126/science.1672776. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Frantz J. D., Strominger J. L., Mulligan R. C. Expression of human class II major histocompatibility complex antigens using retrovirus vectors. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2150–2154. doi: 10.1073/pnas.84.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Singer S. J. Cell biology of cytotoxic and helper T cell functions: immunofluorescence microscopic studies of single cells and cell couples. Annu Rev Immunol. 1989;7:309–337. doi: 10.1146/annurev.iy.07.040189.001521. [DOI] [PubMed] [Google Scholar]
- Liebowitz D., Kopan R., Fuchs E., Sample J., Kieff E. An Epstein-Barr virus transforming protein associates with vimentin in lymphocytes. Mol Cell Biol. 1987 Jul;7(7):2299–2308. doi: 10.1128/mcb.7.7.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz D., Wang D., Kieff E. Orientation and patching of the latent infection membrane protein encoded by Epstein-Barr virus. J Virol. 1986 Apr;58(1):233–237. doi: 10.1128/jvi.58.1.233-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. P., Staunton D., Thorley-Lawson D. A. Epstein-Barr virus-encoded protein found in plasma membranes of transformed cells. J Virol. 1985 Sep;55(3):710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G., Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975 Jul;22(4):276–284. [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K., Klein G. Phenotypic and cytogenetic characteristics of human B-lymphoid cell lines and their relevance for the etiology of Burkitt's lymphoma. Adv Cancer Res. 1982;37:319–380. doi: 10.1016/s0065-230x(08)60886-6. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Patarroyo M., Beatty P. G., Nilsson K., Gahmberg C. G. Identification of a cell-surface glycoprotein mediating cell adhesion in EBV-immortalized normal B cells. Int J Cancer. 1986 Oct 15;38(4):539–547. doi: 10.1002/ijc.2910380414. [DOI] [PubMed] [Google Scholar]
- Patarroyo M., Clark E. A., Prieto J., Kantor C., Gahmberg C. G. Identification of a novel adhesion molecule in human leukocytes by monoclonal antibody LB-2. FEBS Lett. 1987 Jan 5;210(2):127–131. doi: 10.1016/0014-5793(87)81321-2. [DOI] [PubMed] [Google Scholar]
- Patarroyo M., Gahmberg C. G. Phorbol 12,13-dibutyrate enhances lateral redistribution of membrane glycoproteins in human blood lymphocytes. Eur J Immunol. 1984 Sep;14(9):781–787. doi: 10.1002/eji.1830140904. [DOI] [PubMed] [Google Scholar]
- Patarroyo M., Makgoba M. W. Leucocyte adhesion to cells. Molecular basis, physiological relevance, and abnormalities. Scand J Immunol. 1989 Aug;30(2):129–164. doi: 10.1111/j.1365-3083.1989.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Patarroyo M., Yogeeswaran G., Biberfeld P., Klein E., Klein G. Morphological changes, cell aggregation and cell membrane alterations caused by phorbol 12,13-dibutyrate in human blood lymphocytes. Int J Cancer. 1982 Dec 15;30(6):707–717. doi: 10.1002/ijc.2910300606. [DOI] [PubMed] [Google Scholar]
- Prieto J., Takei F., Gendelman R., Christenson B., Biberfeld P., Patarroyo M. MALA-2, mouse homologue of human adhesion molecule ICAM-1 (CD54). Eur J Immunol. 1989 Sep;19(9):1551–1557. doi: 10.1002/eji.1830190906. [DOI] [PubMed] [Google Scholar]
- Shope T., Dechairo D., Miller G. Malignant lymphoma in cottontop marmosets after inoculation with Epstein-Barr virus. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2487–2491. doi: 10.1073/pnas.70.9.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Springer T. A. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989 May 4;339(6219):61–64. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- Takei F. Inhibition of mixed lymphocyte response by a rat monoclonal antibody to a novel murine lymphocyte activation antigen (MALA-2). J Immunol. 1985 Mar;134(3):1403–1407. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Molecular complexity of leukocyte surface glycoproteins related to the macrophage differentiation antigen Mac-1. J Exp Med. 1981 Nov 1;154(5):1517–1524. doi: 10.1084/jem.154.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Wang F., Gregory C., Rickinson A., Larson R., Springer T., Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988 Nov;62(11):4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C., Sample C., Rowe M., Liebowitz D., Murray R., Rickinson A., Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990 May;64(5):2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]