Abstract
Obesity results from a number of factors including socio-environmental influences and rodent models show that several different stressors increase the preference for calorically dense foods leading to an obese phenotype. We present here a non-human primate model using socially housed adult female macaques living in long-term stable groups given access to diets of different caloric density. Consumption of a low fat (LFD; 15% of calories from fat) and a high fat diet (HFD; 45% of calories from fat) was quantified by means of a custom-built, automated feeder that dispensed a pellet of food when activated by a radiofrequency chip implanted subcutaneously in the animal’s wrist. Socially subordinate females showed indices of chronic psychological stress having reduced glucocorticoid negative feedback and higher frequencies of anxiety-like behavior. Twenty-four hour intakes of both the LFD and HFD were significantly greater in subordinates than dominates, an effect that persisted whether standard monkey chow (13% of calories from fat) was present or absent. Furthermore, although dominants restricted their food intake to daylight, subordinates continued to feed at night. Total caloric intake was significantly correlated with body weight change. Collectively, these results show that food intake can be reliably quantified in non-human primates living in complex social environments and suggest that socially-subordinate females consume more calories, suggesting this ethologically relevant model may help understand how psychosocial stress changes food preferences and consumption leading to obesity.
Keywords: automated feeders, food intake, social subordination, rhesus monkey
INTRODUCTION
Rates of obesity have been increasing rapidly in the US with 32% of adults now considered obese (1) and 31% of children and adolescents are at risk for obesity (2). Although obesity may result from a number of factors, socio-environmental influences likely play a significant role in its etiology (3, 4). Recently, a number of studies using rodent models have shown that chronic restraint stress (5) or an elevation in corticosterone can increase the preference for calorically-dense foods, produce a re-distribution of fat to abdominal stores and decrease insulin sensitivity (6–8). Because a signal from the accumulating fat mass may attenuate the neuroendocrine response to new stressors (5) and the consumption of these calorically dense foods may activate reward pathways in mesolimbic regions of the brain (9, 10), these data lend empirical evidence for the popular notion that comfort foods provide relief from environmental stressors while at the same time increasing the risk of developing obesity (11–13). Despite these compelling data, the relations between psychosocial stress, diet, and metabolism is complex. Typically, restraint stress (14, 15) or the stress associated with social subordination in a visible burrow system (16) decreases food intake and body weight relative to non-stressed subjects regardless if animals are maintained on a low fat or a high fat diet (16, 17). The attenuation in food intake is reversed following the stressor (17, 18). Following removal from the visible burrow system, the increase in body weight in the previously subordinate animals is due to an accumulation of visceral fat (18), particularly in rats consuming a high fat diet (16). This effect is thought to be due to a sustained stressed-induced increase in glucocorticoids (19).
Even though these data show that exposure to chronic stressors has a significant impact on metabolism that can lead to the emergence of an obese phenotype in rats (6, 7), rodent models indicate that animals exposed to stressors nevertheless eat less than non-stressed animals (16, 17). By contrast, excess consumption of calories is an essential component for the development of obesity in human populations and it has been suggested that individuals chronically exposed to stressors do consume excess calories (12). Indeed, in exception to the rodent models of stress and changes in adiposity/caloric consumption, group housing Syrian hamsters produces obesity in females (20). Furthermore, in a model that more closely represents the tendency of humans to increase body food intake when stressed, male Syrian hamsters show increased food intake and an accumulation of visceral mesenteric fat in response to a resident-intruder paradigm (21, 22). Although this model shows the impact of an acute psychosocial stressor on food intake, the use of socially-housed macaques living in long-term stable groups also may illustrate how chronic exposure to psychosocial stress affects ingestion and adiposity. Socially subordinate monkeys characteristically exhibit reduced glucocorticoid negative feedback indicative of chronic stress (23, 24) and a significantly higher incidence of abdominal obesity (25, 26). It is unclear, however, whether this central obesity is due to a redistribution of body fat and/or an increase in calorie consumption. The present study was designed to accomplish two goals. First, to validate a method for quantifying food intake in rhesus monkeys living in complex social environments and, secondly, to use this system to begin to examine how social status affects the consumption of calorically dense diets.
METHODS
Characteristics of subjects
Subjects were female rhesus monkeys (Macaca mulatta) who had been born and raised at the Yerkes National Primate Research Center Field Station at Emory University. Females ranged in age from 14 to 18 years and all had been ovariectomized for at least 6 six years. Females had been subjects in a number of studies assessing hormone replacement (27, 28), but had not been treated with any steroids or selective estrogen receptor modulators for one year prior to the start of this study. Females were housed in one of two small social groups (n = 4 and n = 5). The housing unit provided a total of 27.4 m2 of floor space and 3.1 m of vertical space and was divided. The protocol was approved by the Emory University Animal Care and Use Committee in accordance with the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for Care and Use of Laboratory Animals”.
Social status positions were determined by the outcome of dyadic agonistic interactions (29) and had been stable for six years. In these stable groups, social dominance is maintained not through contact aggression, but rather through harassment and the threat of aggression (30). For this study, agonistic data was obtained at least six times each week for 9 weeks from in the morning or afternoon as a part of routine checks on each group. Each assessment typically lasted 20 minutes. The outcome of these dyadic interactions was tabulated to generate a weekly average of submissive gestures to determine a linear hierarchy (Table 1). There was no ambiguity in the social status positions in these two groups as females ranking 2 through 5 each exhibited unequivocal submission gestures (31), e.g., grimace, squeal, and avoid, at either the approach or directed threat from a more dominant female.
Table 1.
The linear dominance hierarchy of the two groups of adult female rhesus monkeys used in the study was constructed on the basis of unequivocal submissive gestures directed toward another animal. Shown are the frequencies (weekly mean over 12 weeks) of these behaviors exhibited by each female in listed in the “actor” column towards recipients. Note that the most dominant female in each group (Yf-1, Zr2) did not exhibit any submissive behaviors while the most subordinate (Ct4, Vg5) submitted to every animal in the group.
| Group A | |||||
|---|---|---|---|---|---|
| Recipient of Submissive behavior | |||||
| Actor | Yf1 | Bf5 | Jc6 | De3 | Ct4 |
| Yf1 | |||||
| BF5 | 11 | ||||
| Jc6 | 9 | 14 | |||
| De3 | 11 | 16 | 21 | ||
| Ct4 | 19 | 28 | 32 | 26 | |
| Group B | ||||
| Recipient of Submissive behavior | ||||
|---|---|---|---|---|
| Actor | Zr2 | Mu3 | Vh5 | Vg5 |
| Zr2 | ||||
| Mu3 | 8 | |||
| Vh5 | 16 | 32 | ||
| Vg5 | 12 | 29 | 11 | |
Social subordination in macaque groups is a chronic psychosocial stressor (32) and is evidenced by a dysregulation of the HPA axis (23, 33). Consequently, each female was administered a dexamethasone (Dex) suppression test to assess glucocorticoid negative feedback. This test was done prior to the start of the presentation of the experimental diets and while the females were consuming the Purina monkey diet (see below). A blood sample was obtained at 2100 hr followed by an IM injection of Dex (0.25 mg/kg). Subsequent samples were collected at 11, 15, and 18 hours the following day. All females had been trained for conscious venipuncture using procedures previously described (34) to allow for the collection of samples without anesthesia. Samples were assayed for cortisol using a commercially available kit (Diagnostics products Corporation, Webster, TX) as described previously (24). The assay had a inter- and intra-coefficient of variation of 6.1% and 3.4%, respectively. In humans, a decrease in glucocorticoid negative feedback is present in several psychiatric disorders, most notably major depression and anxiety (35) and is believed to reflect decreases in central and pituitary glucocorticoid receptor activity (36, 37).
Automated feeders
Females had access to two feeders in the outside portion of their pens. The automated feeding system is contained in three waterproof boxes. Two boxes each contain a Med Associates ENV-203 pellet dispenser (St. Albans, VT) and AVID 1015 microchip ID reader (Norco CA). The reader is compatible with all AVID and fecava coded microchips. In this study, AVID2004 microchips were implanted subcutaneously in both forearms of each animal. Each ID chip contains a unique nine-digit number that is read by the reader and recorded by the computer. In this way, each individual monkey is identified. The feeder boxes were hung directly on the animal enclosure. A plastic shield protected the boxes and connecting wires from animal manipulation. The AVID 6” ring antenna is located directly in front of the treat cup opening. An opening in the shield allows the monkey to place her hand through the opening and access the pellet cups. As the monkey’s hand breaks the plane of the ring antenna, a single pellet is drops into a custom made cup through a clear polypropylene tube. An optical sensor built into the dispenser detects a pellet passing through the tube. The dispenser must be activated again by the monkey for the next pellet to be dispensed and a 4 second delay was built into the software program controlling the dispenser for the delivery of the next pellet. The feeder boxes are hard wired to the control computer box that is located on the building wall adjacent to the animal pen. The computer records the AVID ID codes and controls the two pellet dispensers. The computer is a compact low-power Via EPIA PE1000G based PC with a 1 GHz C3 CPU, 512MB RAM, a 40GB disk drive and 4 serial ports. A custom built interface drives the pellet dispensers. A thermo-electric cooler is located to the right of the dispenser interface. A thermostat set to 70F controls the cooler. A D-Link DWL-G710 802.11g wireless adaptor provides a network connection for the computer. The computer is controlled remotely using VNC software. A simple Visual Basic program monitors the AVID ID readers and controls the dispensers. The program outputs a log of AVID ID numbers recorded by the microchip reader and the time they were recorded driven by the optical sensor detected the pellet passing from the dispenser. The program also monitors the pellet dispensers and will report empty or jammed conditions. Thus, the system records intake of a diet by individual members of a group 24 hours a day, 7 days a week.
Experimental design
Groups were given access to two different experimental diets for 21 days each, with a 3 week period separating the diet presentation during which time they were fed only standard low fat monkey chow. One group had access to the low fat diet (LFD) followed by the high fat diet (HFD) whereas the order was reversed for the other group. The experimental diets were obtained from Research Diets (New Brunswick NJ). The kcal distribution for the LFD (#D05080902) was 20% protein, 65% carbohydrate, and 15% fat while the HFD (D05080901) was 20% protein, 35% carbohydrate, and 45% fat. Differences in fat content were achieved by the addition of lard. Both diets were banana flavored. The LFD had 3.90 kcal/gm while the HFD had 4.62 kcal/gm. Monkeys had continuous access to the feeders except for approximately 45 minutes each day when the outside portion of their pens was sanitized. Following the three-week feeding sessions, each female was given solitary access to the LFD and the HFD on separate one-hour occasions to determine if consumption of the experimental diets during a solitary feeding session was similar to that when all group members were present. Other group members were locked in the indoor portion of their pens during the solitary feeding periods. These solitary feeding sessions occurred between 1300 and 1500 hr corresponding to the time of the day when they typically received their afternoon allotment of Purina chow.
During both three-week periods and solitary sessions, animals also had ad libitum access to the standard monkey diet (#5048; Ralston Purina Company, St. Louis MO). The monkey chow had 3.22 kcal/gm and was comprised of 18% protein, 69% carbohydrate, and 13% fat (by calories). Of the total 2.18 kcal/gram derived from carbohydrates, fiber comprised the greatest component (2.05) for the Purina diet whereas sugars comprised the greatest component for the experimental LFD (2.39 out of 2.53 kcal/gram) and HFD (1.16 out of 1.61 kcal/gm). The Purina diet was presented to the monkeys by filling a bin attached to their caging, although the animals ad libitum access. Consequently, consumption of the Purina diet was not quantified. The rationale for supplementing the animal’s normal diet with the LFD and HFD rather than restricting them to a specific diet was based on observations that male laboratory rats given the choice of eating a HFD or LFD show a greater attenuation in stress responsivity compared to rats restricted to one diet (38). Importantly, this situation also resembles the choice people have to consume foods with different caloric densities. Nevertheless, in order to determine whether the availability of the Purina diet changed the pattern of ingestion of the experimental diets, we compared diet consumption during one week for the LFD and the HFD when the Purina diet was available or unavailable. Animals were weighed at the start and end of the LFD or HFD availability. In addition, females were weighed during a comparable interval when only the standard monkey diet was available. This interval started two-weeks after the completion of second experimental diet session.
During all feeding sessions, animals received a daily supplement of food enrichment, in accordance with the Institution’s animal husbandry standard operating procedure. This enrichment consisted of a daily piece of fruit (e.g., orange, apple, pear, banana) or vegetable (e.g., sweet potato, celery, carrots) and scratch (e.g., mixture of cracked corn, sunflower seeds, peanuts, oats, and cereal). This food enrichment was spread throughout the floor of the housing unit by the animal care staff to minimize competition so that each animal received a piece of fruit or vegetable and was able to forage for scratch. Calorie consumption from these enrichment foods was not quantified and we assume all animals ate the same amount. On average, a piece of fruit or vegetable would provide approximately 15 kcal/kg while the scratch enrichment would provide a maximum of 30 kcal/kg.
Behavioral data were also collected during the period when the Purina diet was unavailable and the monkeys only had access to the HFD or the LFD. Using a standard ethogram (28), observations sessions were 45 minutes to one hour and were done in both the morning and afternoon, totaling 10 hours for each diet. Data were collected as a group scan, with focus on affiliative (proximity, grooming) and agonistic (open mouth threat, bite, slap/grab, grimace, squeal, avoid). In addition, anxiety-like behavior (body shake, scratch self, yawn, and orally groom self) also was recorded. These behaviors are used to assess anxiety in monkeys, as their occurrence is reduced by administration of anxiolytics (39, 40). On a given day, the sessions were timed to coincide with distribution of the food enrichment. The behavior of the female at the automated feeding dispenser also was recorded to determine whether she consumed a pellet that was delivered and whether a higher-ranking female displaced her from the feeder and consumed the pellet she had obtained. Finally, although it was not possible to quantify the amount consumed, note was made whether each animal was able to obtain a piece of fruit/vegetable and forage for scratch.
Data analyses
Data were analyzed by an analysis of variance. Data were transformed before analysis to normalize the variances. Given the small sample size, specific ranks were combined to compare dominant (ranks 1 and 2; n = 4) to subordinate females (ranks 3 through 5; n = 5) using previously described conventions (26). Thus, main effects were social status (dominant vs. subordinate), diet (LFD vs. HFD), availability of the Purina diet, and time (weeks and time of day). For ease of presentation, time of day was divided into daytime (0600 – 1759 hr) and nighttime (1800 – 0559 hr). Bonferroni adjustment (p = .05/n) was used for pair wise comparisons at each time point. In addition, linear regression was used to show the relation between an outcome measure and the specific rank of an animal. All statistical tests with a p ≤ 0.05 were considered significant.
RESULTS
Characteristics of the feeder system
Throughout the course of the study, the output log listed a valid ID chip number, corresponding to an individual animal in the study. In no instance was an invalid ID number listed in the output log. In order to verify that a single pellet was consistently dispensed when the feeder was activated, research personnel activated each feeder with a chip and counted the number of times more than one pellet was dispensed. Of the 112 attempts, a single pellet was dispensed in 109 cases while 2 pellets were dispensed in the other 3 (2.7% of total). In another trial, feeders were filled with a known number of pellets each. After 3 hours of feeding by the animals, the number of pellets remaining in each feeder was subtracted from the number at the start yielding the number consumed. The count of pellets consumed (169) was 1.1% higher than the number indicated on the the computer log file (167). Thus, the feeding system accurately identified the animal that activates the feeder and delivered a single pellet between 97.5 and 98.9% of the time. In the rare instances where more than one pellet was delivered, dust from the pellet likely blocked the optical sensor in the Med Associates dispenser, allowing a second pellet to drop before resetting. Thus, cleaning the optical sensor with a can of compressed air was done multiple times during the day.
In addition, behavioral observation data were obtained that exclusively recorded the an animal’s consumption of a pellet obtained from the feeder to match it to the computer log file. During the four hours of observation, the computer recorded 411 individual feeding bouts. This compared to 358 bouts recorded by the observers or a 12.9% underestimation. Notes made during these observations indicated that an animal activated the feeder multiple times during a session before placing the pellets in her mouth. Importantly, the animal observed to be eating at a specific time point from a specific feeder matched the computer-identified monkey in 100% of the cases.
Social status and glucocorticoid negative feedback
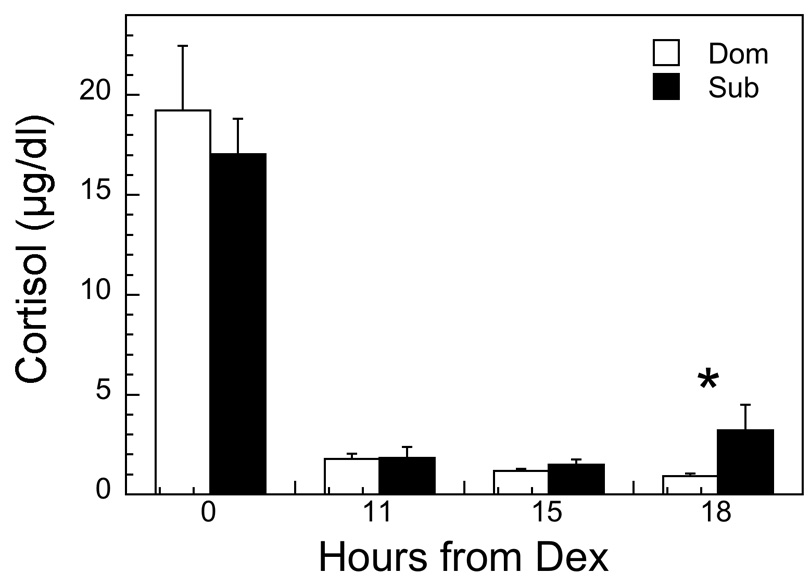
A Dex suppression test was performed to determine whether the subordinate females used in the present study showed reduced glucocorticoid negative feedback, characteristic of socially subordinate macaques. The response to Dex varied significantly between dominant and subordinate females (Figure 1; F3, 21 = 4.83, p = 0.01). Although plasma concentrations of cortisol were not statically different between groups at baseline or at 11 and 15 hr following Dex, levels had increased significantly in subordinate animals by 18 h following Dex. These data indicate that glucocorticoid negative feedback is less efficacious in subordinate females.
Figure 1.
Mean ± sem plasma cortisol concentrations aligned from hours from a dexamethasone (Dex) injection. An asterisk indicates a significant difference (P < 0.05) between dominant (open bar) and subordinate females (black bar) at a specific time point.
Diet intake with Purina diet available
Consumption of both the LFD and HFD was significantly affected by social status (Figure 2; F1, 7 = 7.81, p = 0.03) with subordinate females consuming more kcal per day in each of the three weeks compared to dominant animals. This significantly greater intake by subordinate females did not depend on whether the LFD or HFD was available, as there was no status by diet interaction (F 1, 7 = 0.06, p = 0.90). Furthermore, this effect of status on diet consumption did not vary across the three weeks (status by week interaction: F 1, 7 = 1.03, p = 0.83). Consumption of both diets occurred primarily during the daytime rather than at night (F1, 7 = 36.93, p < 0.01). The effect of status was still evident at night, however, as subordinate females nevertheless consumed significantly more of both diets during this time period than did dominant females (Figure 2; F 1, 7 = 6.06, p = 0.04). Although animals consumed more HFD compared with the LFD diet, the differences were not significant (F1, 7 = 3.67, p = 0.10). The order in which females were given access to these two diets did not contribute significantly to the effect of social status (F 1, 5 = 0.52, p = 0.05) nor did the order affect the amount of each diet consumed by all of the animals (F1, 5 = 5.01, p = 0.08) or as a function of the social status (F1, 5 = 0.01, p = 0.96). Finally, the effect of status on energy intake also was evident during the one-hour solitary feeding sessions (Figure 3), as the correlation between social status rank and kcal consumed was significant for both the LFD (r7 = 0.76, p = 0.02) and HFD (r7 = 0.70, p = 0.04) showing that the lower the rank, the greater the energy intake.
Figure 2.
Mean ± SEM kcal per kg per day consumed by dominant and subordinate females during three-week access to a LFD and a three-week access to a HFD diet. Data illustrate daytime (0600 – 1759 hr) and nighttime (1800 – 0559 hr) food intake. An asterisk indicates values at a specific time point are significantly different between dominant and subordinate females (p < 0.05).
Figure 3.
The linear relation, reflected by the coefficient of determination between a female’s social rank (1 through 5) and the number of kcal consumed during solitary access to a LFD (open symbol) and access to a HFD diet (closed symbol).
Consumption of the diets decreased over the three-week period (F 2, 14 = 10.82, p < 0.01), with intake greater during Week 1 compared to Week 3 (p < 0.05). Overall, intake amounts stabilized by Week 2, as calories consumed during Weeks 2 and 3 were not significantly different. Furthermore, this pattern was significantly influenced by status and time of day (F 2, 14 = 5.05, p = 0.02), as the decrease was less evident in dominant females at night.
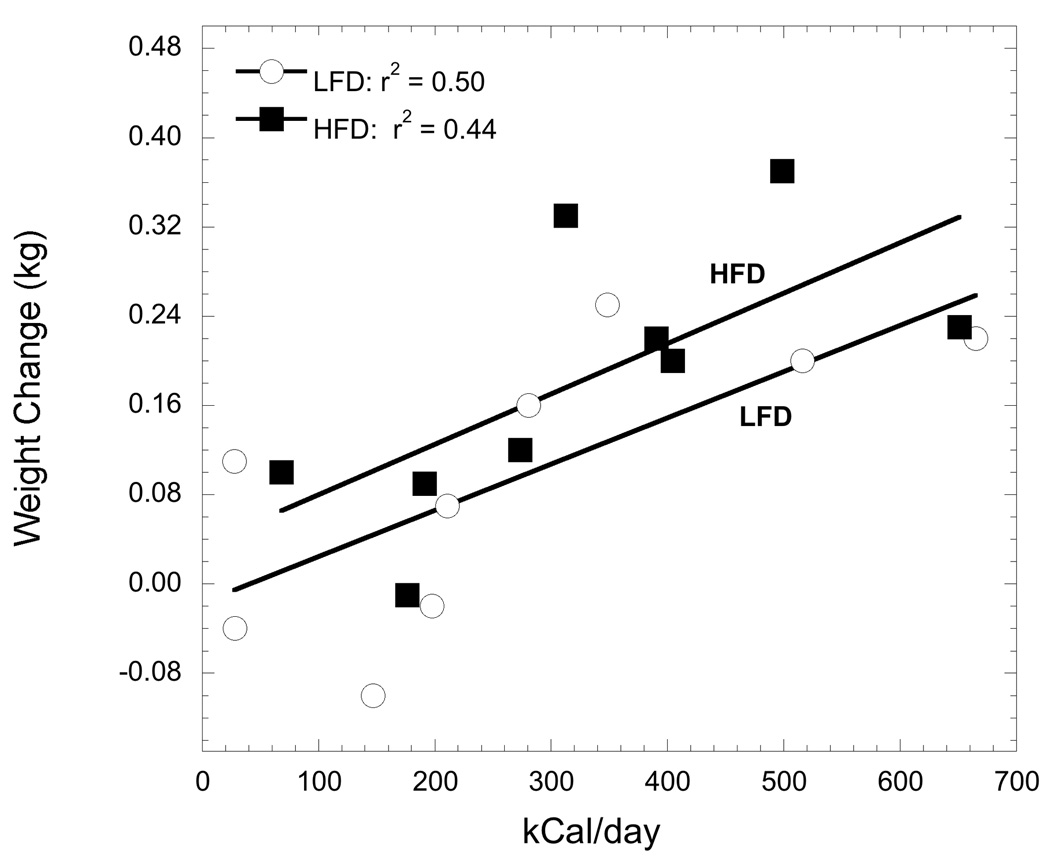
The change in body weight during each 3-week period was compared to a comparable period when only the Purina monkey chow was available (Table 2). Both dominant and subordinate females gained significantly more weight during the LFD and HFD feeding periods compared with the control period when only the standard monkey chow was available (F2, 14 = 4.51, p = 0.03). There was no effect of status on the percent change in weight during the three feeding phases (F2, 14 = 0.50, p = 0.55). The amount of kcal consumed, independent of social status, significantly predicted the amount of weight gain during the LFD (r7 = 0.71, p = 0.03) and HFD sessions (r7 = 0.67, p = 0.05; Figure 4).
Table 2.
Mean ± sem body weight (kg) at the start of the control (Purina diet only), LFD, and HFD feeding period and the percent change in body weight at the end of the three week period. Different superscripts indicate the change in weight was significantly different between feeding phases.
| Social Status | Control | LFD | HFD | |||
|---|---|---|---|---|---|---|
| Start | Percent Change | Start | Percent Change | Start | Percent Change | |
| Dominant | 9.15 ± 0.96 | 0 ± 0%a | 9.09 ± 0.95 | 0.36 ± 0.45%b | 9.09 ± 0.93 | 1.65 ± 0.99%b |
| Subordinate | 8.19 ± 0.69 | 0 ± 0.01%a | 8.31 ± 0.68 | 1.58 ± 0.77%b | 8.21 ± 0.76 | 2.23 ± 0.61%b |
Figure 4.
The linear relation, reflected by the coefficient of determination, between the change in body weight and the number of kcal consumed by a female during three-week access to a LFD (open symbol) and a three-week access to a HFD diet (closed symbol).
Diet intake in the presence and absence of the Purina monkey chow
Females consumed significantly more of the LFD and HFD when Purina was unavailable (Figure 5; F1, 7 = 37.75, p < 0.01). Importantly, subordinate females consumed significantly more of the diets regardless of whether the Purina diet was available or not (F 1, 7 = 9.75, p = 0.02), but the availability of Purina chow did not affect the effect of social status on diet intake (no status by availability interaction, F 1, 7 = 0.78, p = 0.41). The pattern of significantly greater diet intake at night by subordinate compared to dominant females (F 1, 7 = 16.23, p < 0.01) was not affected by the availability of the Purina diet (F 1, 7 = 0.63, p = 0.45). Finally, the preference for the HFD and LFD was significantly affected by Purina availability (F 1, 7 = 9.34, p = 0.02), as females consumed significantly more of the HFD compared with the LFD when the Purina diet was unavailable, but not when it was available. This change in preference was observed for both dominant and subordinate females, as there was no interaction between status, diet, and Purina availability (F 1, 7 = 0.02, p = 0.87).
Figure 5.
Mean ± sem kcal per kg per day consumed of the LFD and the HFD by dominant and subordinate females when Purina monkey chow was available ad libitum (right panel) and when it was unavailable (left panel). Each of the four phases (LFD alone or with the Purina diet and HFD alone or with the Purina diet) were one week in length. Data illustrate daytime (0600 – 1759 hr) and nighttime (1800 – 0559 hr) food intake. An asterisk indicates values at a specific time point are significantly different between dominant and subordinate females (p < 0.05). Significantly more of the LFD and HFD were consumed when the Purina diet was unavailable.
Behavior and Diet
There were no significant differences between dominant and subordinate females nor a significant status by diet interaction for proximity for rates of social and anxiety-like behavior exhibited by females during the LFD and HFD phases when the Purina diet was unavailable (Table 3; F1, 7 = 0.39, p = 0.55, F1, 7 = 0.15, p = 0.71), or for groom frequencies (F1, 7 = 0.54, p = 0.49, F1, 7 = 3.36, p = 0.11) as well as the amount of time females spent in proximity (F1, 7 = 0.04, p = 0.85, F1, 7 = 3.45, p = 0.10) or grooming (F1, 7 = 0.21, p = 0.66, F1, 7 = 2.53, p = 0.16). In contrast, rates of anxiety-like behavior were significantly higher in subordinate compared with dominant females (F1, 7 = 29.62, p < 0.01) and this pattern was similar during both the LFD and HFD (F1, 7 = 0.18, p = 0.68).
Table 3.
Mean ± sem rates of social (initiate proximity or groom) and anxiety-like behavior for dominant (n = 4) and subordinate females (n = 5) in the morning and afternoon during when the LFD and the HFD was available. Behaviors with different superscripts indicate significant differences between dominant and subordinate females.
| Dominant | Subordinate | |||
|---|---|---|---|---|
| Behavior | LFD | HFD | LFD | HFD |
| Initiate Proximity (per hr) | 5.00 ± 0.77 | 5.56 ± 1.21 | 5.30 ± 0.67 | 6.55 ± 1.08 |
| Initiate Groom (per hr) | 1.56 ± 0.71 | 1.06 ± 0.50 | 1.40 ± 0.63 | 2.30 ± 0.45 |
| Proximity Duration (min/hr) | 8.66 ± 1.70 | 8.31 ± 2.52 | 6.16 ± 1.52 | 11.78 ± 2.26 |
| Groom duration (min/hr) | 5.21 ± 1.74 | 4.17 ± 2.01 | 2.11 ± 1.56 | 5.26 ± 1.79 |
| Anxiety-behavior (per hr) | 4.06 ± 0.41a | 5.12 ± 0.93a | 7.50 ± 0.37b | 9.15 ± 0.84b |
The dominant females rarely displaced subordinate females from the feeders or took a pellet of diet obtained by the subordinate animal. On average, subordinate females acquired 745 pellets during the time when behavioral observations were done. Pellets that had been obtained by more subordinate females were taken by a more dominant female in 8 cases or 1.07% of the total. This always involved the more dominant female taking the pellet from the cup following activation by the subordinate animal. No instances were observed in which the subordinate female dropped a pellet she had obtained or a more dominant animal physically taking a pellet away from a subordinate female. Subordinate animals were displaced from the feeders by dominant animals even less frequently (0.08%). Uniformly, females placed the pellet in their mouth while standing at the feeder, regardless of social status. Finally, when the observations sessions coincided with the distribution of the food enrichment, it was noted that all females uniformly obtained a piece of fruit and generally foraged for the scratch provided.
DISCUSSION
The results of this study show the utility of the system developed to quantify food intake in socially-housed macaques as well as the relation of social status to energy intake. Specifically in terms of the former, no formalized training was required for the animals to successfully use the feeders. Although the data reported here summarized the pattern of food intake between daylight and nighttime periods, the program is capable of providing finer resolution to ascertain how any number of factors may influence calories ingested throughout a 24-h period. The data show that the feeders correctly identify monkeys having radiofrequency chips and record the number of pellets actually dispensed within approximately 2%. The difference is related to the very rare occurrence of two pellets being dispensed rather than one. This can be minimized by routinely cleaning the optical sensor in the dispensers. Further assessment of the feeders indicated that the number of pellets consumed by individual females was underestimated by 12.9% based on actual observations of females eating pellets when compared to the computer output file. Notes made during these observations suggest the underestimation of the feeding bouts by the observers is due to the animals accumulating pellets in the cups through multiple activations of the dispensers before consuming the pellets. This behavior does not result in other animals “stealing” pellets as this occurred so infrequently (1.1% of the time). Unfortunately, it was difficult to reliably document females accumulating pellets through multiple activations of the system, as the actual delivery of the pellet into the cup could not be seen by the observer (given the location of the feeder to the observation post) nor could the sound of the pellet dropping into the cup be easily heard (due to the ambient noise in the animal housing facility). In addition, it is also difficult to see if a female places one or more pellets in her mouth, underscoring the difficulty in attempting to quantify food intake of socially housed monkeys through behavioral observations alone. Although the discrepancy between the computer log and the observation record could be problematic, the accuracy of the dispenser for delivering a pellet when activated coupled with the system correctly identifying an animal activating the feeder indicate the feeder system can reliably quantify food intake for individual monkeys housed socially. Nevertheless, future use of this feeder system will require that the computer recorded feeding data be periodically compared to actual observations of food consumption to ensure its continual reliability.
The justification for evaluating how social status affects the consumption of high caloric diets was based on the notion that social subordination in macaque societies represents a chronic psychosocial stressor (23, 32, 33, 41). The subordinate females used in the present study had elevated plasma cortisol following a dexamethasone suppression test, indicative of reduced glucocorticoid negative feedback (35–37). Although rates of social behavior were similar between dominant and subordinate females, the frequency of anxiety-like behaviors was significantly higher in subordinate animals, again indicative of chronic stress exposure (42–44). These physiological and behavioral data support the hypothesis that social subordination represents a psychosocial stressor in macaques (41).
The data clearly show that subordinate females consumed significantly more of both the LFD and HFD over the three week feeding periods. These data are compelling for a number of reasons. It is evident that the dominant animals do not restrict the subordinates from accessing the feeders. Although social status differences in access to resources is a characteristic of macaque groups (29) and dominant females could access the feeder at any time, the behavioral observations revealed that dominant animals displaced subordinates from the feeders in fewer than 1% of the cases in which subordinates were feeding. Furthermore, pellets that had been obtained by more subordinate females were taken by a more dominant female in about 1% of the cases. This always involved the more dominant female taking the pellet from the cup following activation of the feeder by the subordinate animal. No instances were observed in which the subordinate female dropped a pellet she had obtained or a more dominant animal physically taking a pellet away from a subordinate female. These data indicate that that food intake as measured by this feeder system may over estimate calories consumed by subordinates by approximately 1%. Based on the data presented here, subordinate animals consumed on average 55% more of the special diets than did dominant females. Thus, an overestimation of 1% of pellet intake for subordinate females does not invalidate the results. It is likely that social status competition for the diets was minimized in this study because the diets were available 24 hours each day and the dominant animals could eat anytime they chose to and, based on the data, these dominant females simply ate less frequently than did subordinate females. This social status-dependent pattern also was evident during the solitary feeding sessions, as social rank significantly predicted calories consumed of both the low fat and high fat diets. Thus, in the presence or absence of more dominant cage mates, subordinate females nevertheless eat more of these diets.
Because the Purina monkey chow was available during the three-week sessions when the high fat or low fat diets were accessible through the feeders, it is possible that the dominant females were eating more of the Purina chow, forcing subordinate animals to consume the special diets. Comparing the calories consumed of the high fat and low fat diets when the Purina chow was available or unavailable, however, showed that the subordinate females ate more of these special diets regardless of whether the Purina chow was present. All animals consumed more of both the high fat and low fat diets when the Purina chow was unavailable but the subordinates continued to consume significantly more during both the daytime and nighttime. These data suggest that dominant animals restricted their intake of these diets compared to subordinate animals. The food enrichment provided to the animals during all feeding sessions likely provided minimal nutrition to the animals compared to the Purina chow and special diets. Observations that coincided with the distribution of the food enrichment indicated all females, regardless of rank, obtained the fruit or vegetable and ate some of the scratch enrichment, indicating that whatever calories were derived from these food was similar among the animals. Although future studies should account for the calories provided by these items, the data nevertheless indicate that socially subordinate females consume more of the high fat and low fat diet than do dominant females.
Restraint stress (14, 15) or the stress associated with social subordination in a visible burrow system (16) decreases food intake and body weight (16, 17). However, food intake is no longer suppressed following the stressor (17, 18) and the subsequent increase in body weight in formally subordinate animals is due to an accumulation of visceral fat (18), particularly in rats consuming a high fat diet (16). Studies of male Syrian hamsters show chronically defeated animals over consume a standard laboratory chow and develop an obese phenotype (21). Although one could argue that this represents a more ethologically relevant stressor than restraint, it is unclear how social defeat is different than that of social subordination in the visible burrow system, other than the intensity of the social interactions. Indeed, the amount of time animals are together is significantly less in the social defeat paradigm (21, 22) compared with the visible burrow system (18). On the other hand, socially-housed Siberian hamsters are fatter (20, 45, 46), suggesting a consequence of social housing may lead to excess calorie consumption and/or that hamsters, in general respond to stress more similarly to humans (and macaques here) by increasing food intake and body fat. Indeed, Syrian hamsters given foot shock increase food intake and body fat as well (22). The data from the present study are the first to quantify food intake in socially housed female macaques and would suggest that in this context, social subordination could produce an obese phenotype resulting from a greater consumption of calories. This effect, however, may be dependent on the diet available. These discrepancies between different animal models reflect the complex relation between psychosocial stress, food intake and species underscoring the importance of conducting more research to better understand what situations lead to reduced food intake and decreased body fat and what circumstances produce excess food consumption and increased body fat.
Based on data from laboratory rat models (8), we predicted that the high fat diet would be preferred, particularly by subordinate females. Given the small sample size and individual variability, however, the observed greater consumption of this diet approached but was not statistically significant (p = 0.09). An unanticipated outcome was the significantly greater consumption of the low fat diet by the subordinate compared to dominant females. At this time, it is difficult to determine what may account for this. Although the experimental low fat diet used in this study had slightly more fat than the Purina monkey chow (15 vs 13% of kcal), it is unlikely that this accounts for the increased consumption of the special diet. Rather the banana flavor, coupled with the fact that the carbohydrate component is derived from sugars rather than fiber, may make the low fat experimental diet more palatable than the Purina chow. Furthermore, the caloric difference (~0.70 kcal/gm of diet) between the high fat and low fat diet was similar to that of the low fat diet compared to the Purina chow. Thus, assuming the banana flavor can be controlled, the appropriate “low fat control” for future studies may be the Purina chow. Furthermore, as noted in rodent studies (38), in order to fully understand diet preference and consumption patterns related to psychosocial factors associated with social status hierarchies, diets need to be presented simultaneously and total daily calories consumed from a low fat and a high fat diet needs to be quantified.
In summary, the present study shows that food intake can be quantified in rhesus monkeys housed in complex social environments. The data collected as a part of the validation of the automated feeding system show that socially-subordinate female rhesus monkeys consume more calories from these palatable diets throughout a 24 h. The system provides a reliable means to study any number of factors related to food intake, including changes in satiety and orexigenic hormones, food preferences, meal timing and caloric consumption, as well as factors that affect these feeding patterns and the metabolic and physiological consequences. Because psychosocial stress exposure increases the risk of engaging in addictive behaviors (47), and because calorically dense foods can activate dopamine reward pathways (48), the use of this automated feeding system with a rhesus monkey model can help elucidate the complex interaction between psychosocial stress, comfort food ingestion, and obesity.
ACKNOWLEDGEMENTS
The expert technical assistance of Jennifer Whitley, Holly Jarrell, and Dr. Jackie Hoffman contributed significantly to the completion of this project. This project was supported by in part by the STC Program of the National Science Foundation IBN-9876754, HD46501, and RR00165.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Jama. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. Jama. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 3.Brunner EJ, Chandola T, Marmot MG. Prospective effect of job strain on general and central obesity in the Whitehall II Study. Am J Epidemiol. 2007;165:828–837. doi: 10.1093/aje/kwk058. [DOI] [PubMed] [Google Scholar]
- 4.Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev. 2006;27:750–761. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- 5.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 6.la Fleur SE, Akana SF, Manalo SL, Dallman MF. Interaction between corticosterone and insulin in obesity: regulation of lard intake and fat stores. Endocrinology. 2004;145:2174–2185. doi: 10.1210/en.2003-1359. [DOI] [PubMed] [Google Scholar]
- 7.Bhatnagar S, Bell ME, Liang J, Soriano L, Nagy TR, Dallman MF. Corticosterone facilitates saccharin intake in adrenalectomized rats: does corticosterone increase stimulus salience? J Neuroendocrinol. 2000;12:453–460. doi: 10.1046/j.1365-2826.2000.00487.x. [DOI] [PubMed] [Google Scholar]
- 8.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 10.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 11.Dallman MF, La Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids--food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145:2633–2638. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- 12.Epel E, Jimenez S, Brownell K, Stroud L, Stoney C, Niaura R. Are stress eaters at risk for the metabolic syndrome? Ann N Y Acad Sci. 2004;1032:208–210. doi: 10.1196/annals.1314.022. [DOI] [PubMed] [Google Scholar]
- 13.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 14.Marti O, Marti J, Armario A. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav. 1994;55:747–753. doi: 10.1016/0031-9384(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 15.Harris RB, Mitchell TD, Simpson J, Redmann SM, Jr, Youngblood BD, Ryan DH. Weight loss in rats exposed to repeated acute restraint stress is independent of energy or leptin status. Am J Physiol Regul Integr Comp Physiol. 2002;282:R77–R88. doi: 10.1152/ajpregu.2002.282.1.R77. [DOI] [PubMed] [Google Scholar]
- 16.Tamashiro KL, Hegeman MA, Sakai RR. Chronic social stress in a changing dietary environment. Physiol Behav. 2006;89:536–542. doi: 10.1016/j.physbeh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Harris RB, Zhou J, Youngblood BD, Rybkin, Smagin GN, Ryan DH. Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am J Physiol. 1998;275:R1928–R1938. doi: 10.1152/ajpregu.1998.275.6.R1928. [DOI] [PubMed] [Google Scholar]
- 18.Tamashiro KL, Nguyen MM, Fujikawa T, Xu T, Yun Ma L, Woods SC, Sakai RR. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav. 2004;80:683–693. doi: 10.1016/j.physbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Strack AM, Bradbury MJ, Dallman MF. Corticosterone decreases nonshivering thermogenesis and increases lipid storage in brown adipose tissue. Am J Physiol. 1995;268:R183–R191. doi: 10.1152/ajpregu.1995.268.1.R183. [DOI] [PubMed] [Google Scholar]
- 20.Meisel RL, Hays TC, Del Paine SN, Luttrell VR. Induction of obesity by group housing in female Syrian hamsters. Physiol Behav. 1990;47:815–817. doi: 10.1016/0031-9384(90)90002-l. [DOI] [PubMed] [Google Scholar]
- 21.Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1284–R1293. doi: 10.1152/ajpregu.00437.2005. [DOI] [PubMed] [Google Scholar]
- 22.Solomon MB, Foster MT, Bartness TJ, Huhman KL. Social defeat and footshock increase body mass and adiposity in male Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2007;292:R283–R290. doi: 10.1152/ajpregu.00330.2006. [DOI] [PubMed] [Google Scholar]
- 23.Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Sapolsky RM. Are subordinates always stressed? a comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- 24.Wilson ME, Pazol K, Legendre A, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic - hypothalamic - pituitary - adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26 doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- 25.Shively CA, Clarkson TB. Regional obesity and coronary artery atherosclerosis in females: a non-human primate model. Acta Med Scand Suppl. 1988;723:71–78. doi: 10.1111/j.0954-6820.1987.tb05930.x. [DOI] [PubMed] [Google Scholar]
- 26.Shively CA, Grant KA, Ehrenkaufer RL, Mach RH, Nader MA. Social stress, depression, and brain dopamine in female cynomolgus monkeys. Annals of the New York Academy of Sciences. 1997;807:574–577. doi: 10.1111/j.1749-6632.1997.tb51972.x. [DOI] [PubMed] [Google Scholar]
- 27.Wilson ME, Mook D, Graves F, Felger J, Bielsky IF, Wallen K. Tamoxifen is an estrogen antagonist on gonadotropin secretion and responsiveness of the hypothalamic-pituitary- adrenal axis in female monkeys. Endocrine. 2003;22:305–315. doi: 10.1385/ENDO:22:3:305. [DOI] [PubMed] [Google Scholar]
- 28.Mook D, Felger J, Graves F, Wallen K, Wilson ME. Tamoxifen fails to affect central serotonergic tone but increases indices of anxiety in female rhesus macaques. Psychoneuroendocrinology. 2005;30:273–283. doi: 10.1016/j.psyneuen.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein IS. Dominance, aggression and reproduction in primate societies. J Theor Biol. 1976;60:459–472. doi: 10.1016/0022-5193(76)90072-2. [DOI] [PubMed] [Google Scholar]
- 30.Bernstein IS, Gordon TP, Rose RM. Aggression and social controls in rhesus monkey (Macaca mulatta) groups revealed in group formation studies. Folia Primatol (Basel) 1974;21:81–107. doi: 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- 31.Altmann SA. A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Ann N Y Acad Sci. 1962;102:338–435. doi: 10.1111/j.1749-6632.1962.tb13650.x. [DOI] [PubMed] [Google Scholar]
- 32.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 33.Wilson ME, Legendre A, Pazol K, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic-hypothalamic-pituitary-adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26:89–97. doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- 34.Walker ML, Gordon TP, Wilson ME. Reproductive performance in capture-acclimated female rhesus monkeys (Macaca mulatta) J Med Primatol. 1982;11:291–302. [PubMed] [Google Scholar]
- 35.Heuser I. Anna-Monika-Prize paper. The hypothalamic-pituitary-adrenal system in depression. Pharmacopsychiatry. 1998;31:10–13. doi: 10.1055/s-2007-979288. [DOI] [PubMed] [Google Scholar]
- 36.de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Calfa G, Kademian S, Ceschin D, Vega G, Rabinovich GA, Volosin M. Characterization and functional significance of glucocorticoid receptors in patients with major depression: modulation by antidepressant treatment. Psychoneuroendocrinology. 2003;28:687–701. doi: 10.1016/s0306-4530(02)00051-3. [DOI] [PubMed] [Google Scholar]
- 38.la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146:2193–2199. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- 39.Kalin NH. Nonhuman primate studies of fear, anxiety, and temperament and the role of benzodiazepine receptors and GABA systems. J Clin Psychiatry. 2003;64 Suppl 3:41–44. [PubMed] [Google Scholar]
- 40.Troisi A. Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress. 2002;5:47–54. doi: 10.1080/102538902900012378. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan JR, Manuck SB. Ovarian dysfunction, stress, and disease: a primate continuum. ILAR. 2004;45:89–115. doi: 10.1093/ilar.45.2.89. [DOI] [PubMed] [Google Scholar]
- 42.Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, Webster EL, Atkinson AJ, Schulkin J, Contoreggi C, Chrousos GP, McCann SM, Suomi SJ, Higley JD, Gold PW. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6079–6084. doi: 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Owens MJ, Nemeroff CB. The role of corticotropin-releasing factor in the pathophysiology of affective and anxiety disorders: laboratory and clinical studies. Ciba Foundation Symposium. 1993;172:296–308. doi: 10.1002/9780470514368.ch15. discussion -16. [DOI] [PubMed] [Google Scholar]
- 45.Bartness TJ. Photoperiod, sex, gonadal steroids, and housing density affect body fat in hamsters. Physiol Behav. 1996;60:517–529. doi: 10.1016/s0031-9384(96)80027-8. [DOI] [PubMed] [Google Scholar]
- 46.Borer KT, Pryor A, Conn CA, Bonna R, Kielb M. Group housing accelerates growth and induces obesity in adult hamsters. Am J Physiol. 1988;255:R128–R133. doi: 10.1152/ajpregu.1988.255.1.R128. [DOI] [PubMed] [Google Scholar]
- 47.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]