Abstract
Genitourinary tract inflammation/ailments affect the quality of life and health of a large segment of society. In recent years, studies have demonstrated strong anti-oxidant effects of green tea and its associated polyphenols in inflammatory states. This in vitro study examined the antioxidant capabilities (and putative mechanisms of action) of green tea extract (GTE), polyphenon-60 (PP-60, 60 % pure polyphenols), (−)-epicatechin-3-gallate (ECG) and (−)-epigallocatechin-3-gallate (EGCG) in normal/malignant human bladder cells following catechin treatment ± 1 mM H2O2 (oxidative agent). Cell viability, apoptosis and reactive oxygen species (ROS) formation were evaluated. Our results showed that H2O2 exposure significantly reduced normal (UROtsa) and high-grade (TCCSUP, T24) bladder cancer (BlCa) cell viability compared with control-treated cells (p < 0.001). No affect on low-grade RT4 and SW780 BlCa cell viability was observed with exposure to H2O2. Compared to H2O2-treated UROtsa, treatment with PP-60, ECG and EGCG in the presence of H2O2 significantly improved UROtsa viability (p < 0.01), with strongest effects evoked by ECG. Additionally, though not as effective as in UROtsa cells, viability of both high-grade TCCSUP and T24 BlCa cells, in comparison to H2O2 -treated cells, were significantly improved (p < 0.01) by treatment with PP-60, ECG, and EGCG in the presence of H2O2. Overall, our findings demonstrate that urothelium cell death via H2O2-induced oxidative stress is mediated, in part, through superoxide (O2−·), and potentially, direct H2O2 mechanisms, suggesting that green tea polyphenols can protect against oxidative stress/damage and bladder cell death.
Keywords: Green Tea, Hydrogen Peroxide, Cytoprotection, Polyphenols, Oxidative Stress
Introduction
Green tea, extracted from Camellia sinensis, is a widely consumed beverage throughout the world second only to water (Dreosti, 1996). Green tea contains multiple catechin components, though (−)-epigallocatechin-3-gallate (EGCG; Fig. 1) is the primary catechin accounting for 50–80% in a brewed cup (Khan et al., 2006). ECG [(−)-epicatechin-3-gallate] (Fig. 1) is the second most concentrated catechin component of green tea, and is associated with its anti-inflammatory/anti-oxidant properties (Rice-Evans et al., 1995). Other major catechins found in green tea (Fig. 1) include (−)-epicatechin (EC) and (−)-epigallocatechin (EGC).
Fig. 1.
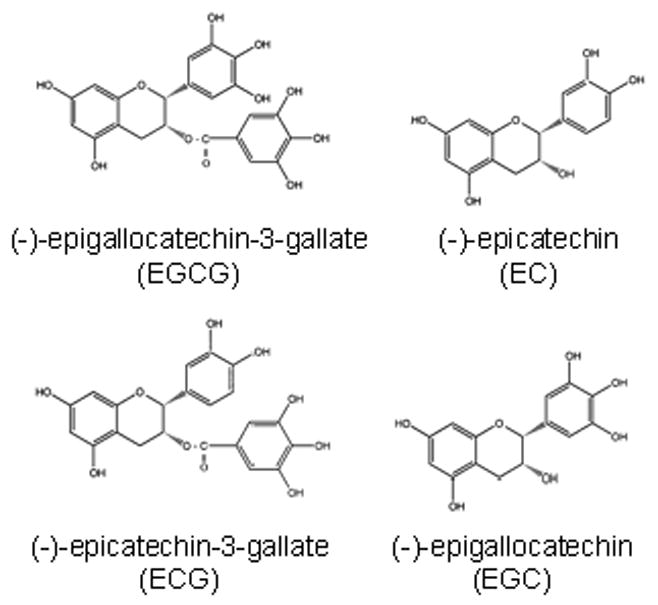
Chemical structures of (−)-epicatechin (EC), (−)-epicatechin-3-gallate (ECG), (−)-epigallocatechin (EGC) and (−)-epigallocatechin-3-gallate (EGCG). ECG and EGCG have strong antioxidant activities and are thought to afford protection against a variety of cancers.
Increased formation and tissue levels of hydrogen peroxide (H2O2), a noxious oxidant and signaling molecule, are observed with inflammation and disease. Elevated concentrations of H2O2, dependent upon diet, lifestyle, and other health factors (Halliwell et al., 2000), can be found in the bladder and lower urinary tract. Epidemiological studies indicate that chronic inflammatory diseases are associated with an increased risk of human malignancies (Lu et al., 2006), including bladder cancer (BlCa) (La Vecchia et al., 1991; Hofseth et al., 1996) and chemical toxicity/irritation.
Though limited in the bladder, previous studies have identified anti-inflammatory/anti-oxidant properties of green tea in both in vivo and in vitro systems (Chan et al., 1997; Katiyar et al., 1999). Indeed, due to its low cost, low cytotoxicity and widespread availability, green tea has enormous potential as a chemopreventative agent for a variety of human diseases. Consequently, because catechin components have been detected at measureable levels in human urine (Yang et al., 1999; Lee et al., 2002), green tea and other herbal supplements may be beneficial and under-utilized dietary resources to modulate lower urinary tract inflammation.
In the present study, normal/malignant (low-/high-grade) human bladder uroepithelial cells were utilized to investigate the anti-oxidant properties of green tea extract (GTE), polyphenon-60 (PP-60; 60 % pure catechins) and two GTE components (ECG and EGCG) following oxidative stress with H2O2. Our data demonstrate that under in vitro conditions, PP-60, ECG and EGCG can afford both normal and tumorigenic human bladder urothelial cells protection (i.e., prevent apoptosis) to various degrees after chemical insult with H2O2. Induction of apoptosis by H2O2 in normal human bladder cells appears to be mediated partially by superoxide (O2−·.) radicals and peroxynitrite (ONOO−), suggesting that catechins have important implications for the successful protection of bladder uroepithelial cells under both physiologic and diseased conditions.
Materials and methods
Chemicals
Green Tea Extract (GTE), obtained from General Nutrition Corporation (Pittsburgh, PA), was a mixture of multiple catechin compounds (14 % polyphenols). Polyphenon-60 (PP-60, CAS registry number: 138988-88-2), ECG (CAS registry number: 1257-08-5) and EGCG (CAS registry number: 989-51-5) were purchased from Sigma Chemical Co. (St. Louis, MO) and were 95–98 % pure as assessed by the commercial source (via HPLC). Hydrogen peroxide (H2O2, 50 % w/v) and 4,5- Dihydroxy-1,3-benzenedisulfonic acid disodium salt (Tiron, superoxide [O2−·] scavenger, anti-oxidant mimic) were also obtained from Sigma Chemical Co. Stock solutions of GTE (1 mg/mL), PP-60 (1 mg/mL), ECG (0.5 mg/mL), EGCG (1 mg/mL) and Tiron (1 mM) were prepared monthly in Dulbecco’s Modified Eagle Media (DMEM, Gibco, Grand Island, NY) under sterile conditions, and stored at 4°C. H2O2 (10 mM) was prepared (diluted in DMEM) on the day of treatment.
Enzymes
Superoxide dismutase-polyethylene glycol (SOD, 1350 U/mg protein) from bovine erythrocytes and Catalase-polyethylene glycol (CAT, 40,000 U/mg protein) from bovine liver were purchased from Sigma Chemical Co. and prepared (diluted in DMEM) on the day of treatment.
Cell Culture
The human bladder transitional cell carcinoma (TCC) cell line TCCSUP was obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured in Minimum Essential Media (MEM, Gibco) with phenol red, supplemented with 10 % (v/v) fetal bovine serum (FBS) and 1 % (v/v) penicillin-streptomycin (PS, Gibco). Human SW780 TCC-derived cells (ATCC) were cultured in Leibovitz’s L-15 Media (Gibco) without phenol red, supplemented with 10 % (v/v) FBS. Human BlCa T24 and RT4 cells (ATCC) were cultured in McCoy’s (Modified) 5A Media (Gibco) with phenol red, supplemented with 10 % (v/v) FBS and 1 % (v/v) PS. Normal human urothelium UROtsa cells were kindly provided by Dr. Scott Garrett (University of North Dakota, USA) and cultured in DMEM (Gibco) without phenol red, supplemented with 5 % (v/v) FBS, 1 % (v/v) PS and 1.5 mM L-glutamine (Gibco). Cell lines were maintained at 37°C in a humidified 5 % CO2 incubator.
Assessment of Cell Viability (XTT)
Bladder cell viability was measured using the Sigma In Vitro Toxicology Assay Kit (XTT) as per manufacturer’s instructions. The XTT-based system is a reliable index to measure mitochondrial activity and thus cell viability/function. Briefly, cells were seeded in triplicate in 24-well plates (Corning, Corning, NY), at a density of 100,000–175,000 cells/well, and grown for 24 hours at 37°C. After reaching 75–80 % confluence, cells were treated with increasing concentrations of GTE, PP-60, ECG and EGCG (0, 10, 20 and 40 μg/mL) with or without 1 mM H2O2 for 1 hour. Cells were then washed with serum-free DMEM and incubated in complete DMEM for 23 hours at 37°C. Following addition of 200 μL XTT reagent diluted in serum-free DMEM (20 hours after initiation of incubation), plates were covered and incubated at 37°C for 4 hours. Supernatants were removed and added to 96-well plates in triplicate, and absorbance measured at 450 nm in a Bio-Tek ELx800 Microplate reader (Winooski, VT). Reduction in cell viability/function was expressed as a percentage of total activity/cell number compared to non-treated control cells (considered 100 % viable).
For mechanistic-based studies, cells (UROtsa only) were treated with CAT (200 U), SOD (200 U), CAT (200 U) + SOD (200 U) or Tiron (1 mM) with or without 1 mM H2O2 for 1 h at 37°C. Viability was assessed as above.
Measurement of Apoptosis (Annexin V)
Apoptosis was probed using the Annexin V detection kit (Molecular Probes, Inc., Eugene, OR) according to the manufacturer’s instructions. Briefly, UROtsa cells (50,000 cells/chamber) were seeded in duplicate in 8-chambered plastic slides (NUNC, Rochester, NY), grown for 24 hours at 37°C, and then treated with ECG (40 μg/mL), EGCG (40 μg/mL) or Tiron (1 mM) with or without 1 mM H2O2 for 1 hour at 37°C. As a positive control, cells were exposed to 500 μM EtOH. Cells were then washed with serum-free DMEM and incubated in complete DMEM for 23 hours at 37°C. After incubation, cells were washed with cold PBS and incubated with 20 μL Annexin V-Alexa 488 conjugate (Invitrogen, Carlsbad, CA) diluted in buffer (pH 7.4) for 30 minutes at 37°C. Finally, cells were washed with buffer, coverslipped and imaged using a bright field/fluorescence microscope (Olympus BX51) and digital camera (Magnafire™, Olympus). Fluorescence, expressed as relative fluorescence units (RFU) per cell, was measured by the area under the curve method following correction for background using Image-Pro Express™ version 4.0 (Media Cybernetics, Silver Spring, MD).
Measurement of Intracellular Reactive Oxygen Species (ROS)
Levels of ROS were quantified with enhanced chemiluminescence using coelenterazine cp (Invitrogen). Coelenterazine is a lipophilic luminophore originally isolated from the coelenterate Aequorea and shown to be a sensitive chemiluminescent probe for measuring O2−· (Duerrschmidt et al., 2006) and ONOO− (Tarpey et al., 1999) in biological systems. Briefly, cells were seeded in triplicate in 60 mm dishes (BD BioSciences, San Jose, CA) at a density of 1.2 × 106 cells/dish, and grown for 24 hours at 37°C. After reaching 75–80 % confluence, cells were treated with EGCG (40 μg/mL) or Tiron (1 mM) with or without 1 mM H2O2 for 5 min at 37°C. As a positive control, cells were exposed to 1 mM H2O2. Following incubation, cells were scraped from each dish, after which time 5 μL coelenterazine cp (1 mM diluted in 100 % MeOH) was added. Cell mixtures were transferred to luminometer cuvettes (BD BioSciences) and chemiluminescence measured in a Berthold AutoLumat Plus Luminometer (Oak Ridge, TN) for 3 minutes (2 second sampling).
Statistical Analysis
Statistical significance was evaluated by one-way analysis of variance (ANOVA) and Bonferroni t post-test using Prism (statistical software) from GraphPad. A level of p < 0.05 was considered significant. Values are reported as mean ± standard error of measure (SEM).
Results
PP-60, ECG and EGCG Catechins Protect Human Bladder Cells From H2O2-Induced Oxidative Stress
To ascertain the protective nature of GTE, PP-60, ECG and EGCG (0–40 μg/mL) viability in normal/malignant human bladder cells exposed to H2O2 for 1 hour was probed. H2O2 is an oxidative agent, which has been shown to induce ROS formation in multiple cell types (Coyle et al., 2006; Li et al., 2001). After exposure, XTT assays were performed to examine agent dose-dependency. Protective effects of GTE, PP-60, ECG and EGCG were examined in five human bladder cell lines (1 normal [UROtsa], 4 tumorigenic [RT4, SW780, TCCSUP, T24]). Exposure of UROtsa (Fig. 2) and high-grade BlCa TCCSUP (Fig. 3A) urothelial cells to 1 mM H2O2 significantly reduced viability compared with control-treated cells (p < 0.01, n=12–16). In contrast, H2O2 exposure moderately reduced high-grade BlCa T24 cell viability (Fig. 3B), in comparison to control cells (p < 0.01, n=12–16).
Fig. 2.

Effects of Green Tea Catechins (PP-60, ECG, EGCG) on Normal Human Bladder UROtsa Cell Viability. UROtsa cells were treated with increasing concentrations of agent in the presence or absence of 1 mM H2O2 and XTT assay performed as described in Materials and methods. Data (means ± SEM) are representative of three independent experiments. *Significant difference at p < 0.01 versus control; #Significant difference at p < 0.01 versus H2O2.
Fig. 3.
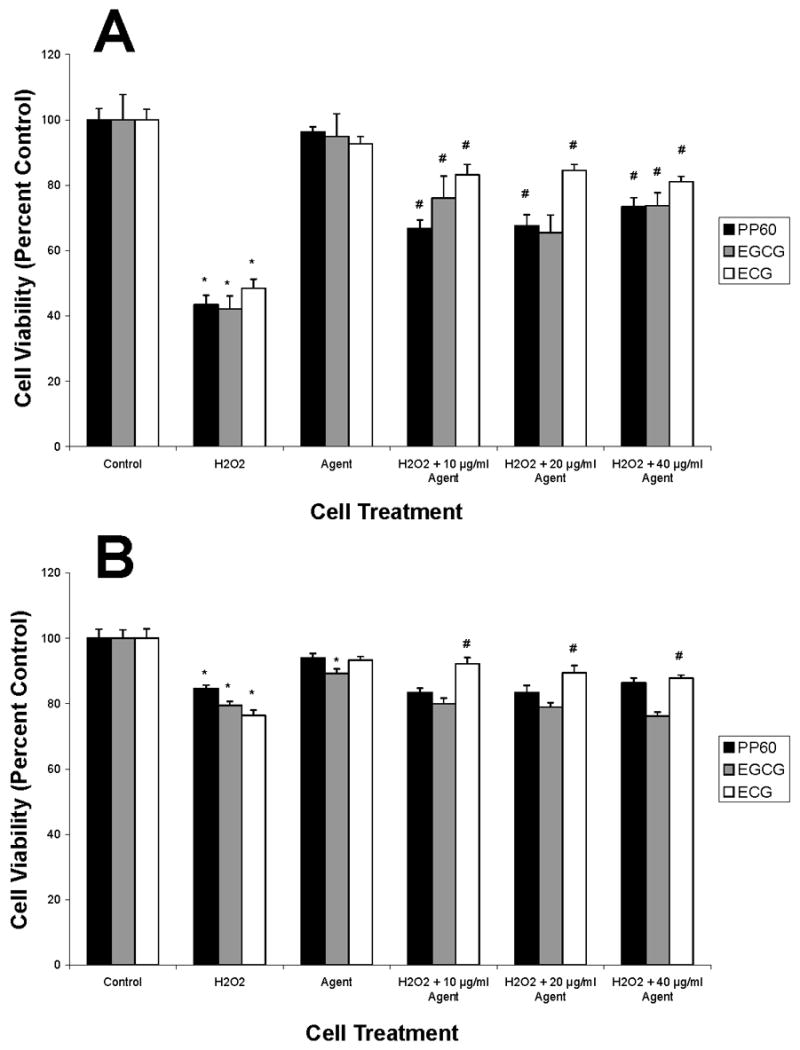
Effects of Green Tea Catechins (PP-60, ECG, EGCG) on High-Grade Human Bladder Cancer TCCSUP (A) and T24 (B) Cell Viability. Cells were treated with increasing concentrations of agent (agent alone is 40 μg/mL) in the presence or absence of 1 mM H2O2 and XTT assay performed as described in Materials and methods. Data (means ± SEM) are representative of three independent experiments. *Significant difference at p < 0.01 versus control; #Significant difference at p < 0.01 versus H2O2.
We also observed that PP-60, ECG and EGCG (10, 20, 40 μg/mL) significantly improved the viability of UROtsa cells exposed to 1 mM H2O2 (p < 0.01, n=12–16) compared to H2O2-treated cells (Fig. 2). GTE, conversely, did not improve the viability of UROtsa cells following treatment with 1 mM H2O2 (data not shown). In low-grade RT4 and SW780 BlCa cells, no loss in viability was observed with H2O2 exposure (data not shown). Survival of high-grade TCCSUP (Fig. 3A) and T24 (Fig. 3B) BlCa cells to H2O2 insult was significantly improved with 10, 20 and 40 μg/mL ECG (p < 0.001, n=12–16). Additionally, PP-60 and EGCG (10, 20, 40 μg/mL) provided protection against H2O2 to TCCSUP cells, but not to T24 cells. From a clinical perspective, it is noteworthy that PP-60, ECG and EGCG afforded normal UROtsa cells significantly greater protection from H2O2 insult compared to both TCCSUP and T24 BlCa cells. ECG and EGCG Catechins Protect Human Bladder Cells From H2O2-Induced Apoptosis
We next determined whether H2O2-mediated loss of UROtsa cell viability is: 1) mediated through apoptosis and 2) modulated by ECG and/or EGCG. Because our studies focused on acute chemical insult, mimicking localized human inflammatory conditions, normal human UROtsa cells were selected for apoptotic analysis. A dose of 40 μg/mL ECG or 40 μg/mL EGCG (1 hour exposure) was selected based upon XTT viability results (Figs. 2, 3). Furthermore, because ECG and EGCG are: 1) the most prevalent polyphenols in green tea, 2) thought to be responsible for most of the anti-inflammatory/cancer-preventative properties of green tea and 3) the most studied and characterized of the green tea catechins, these two agents were utilized exclusively for apoptotic analysis. Apoptosis was confirmed by fluorescent staining with Annexin V (Tarpey et al., 1999) and analysis of cellular characteristics of apoptosis (i.e., cell shrinkage, membrane blebbing).
Fig. 4 shows representative fluorescent images (24 h post-treatment) of Annexin V staining (cell death marker) in treated and control-treated UROtsa bladder cells. Quantitative fluorescence measurement (RFU/cell) revealed increased Annexin V staining in H2O2-treated (Fig. 4B) and EtOH-treated (Fig. 4I) cells, compared to non-treated controls (Fig. 4A). Specifically, in comparison to untreated cells, H2O2-treated (46,000 RFU/cell vs 24,000 RFU/cell) and EtOH-treated (52,000 RFU/cell vs 24,000 RFU/cell) cells averaged approximately 2-fold greater RFU/cell. Phase-contrast imaging also revealed decreased cell density in these two treatment groups (> 3-fold vs non-treated cells). Treatment with either 40 μg/mL ECG (Figs. 4E, 4F) or EGCG (Figs. 4G, 4H) protected UROtsa cells from oxidative stress-induced cell death. Specifically, ECG- and EGCG-treated cells, in the presence of H2O2, averaged 18,000 RFU/cell and 17,000 RFU/cell, respectively, compared to cells treated with catechin agent alone (63,000 RFU/cell and 96,000 RFU/cell, respectively). Similarly, 1 mM Tiron (O2−· scavenger) afforded UROtsa cells strong protection (12,000 RFU/cell vs 46,000 RFU/cell) against H2O2-induced cell death (Figs. 4C, 4D). These results suggest a duality of catechin action, with contributions to both apoptosis (in non-stressed cell populations) and cell survival (in stressed cell populations).
Fig. 4.
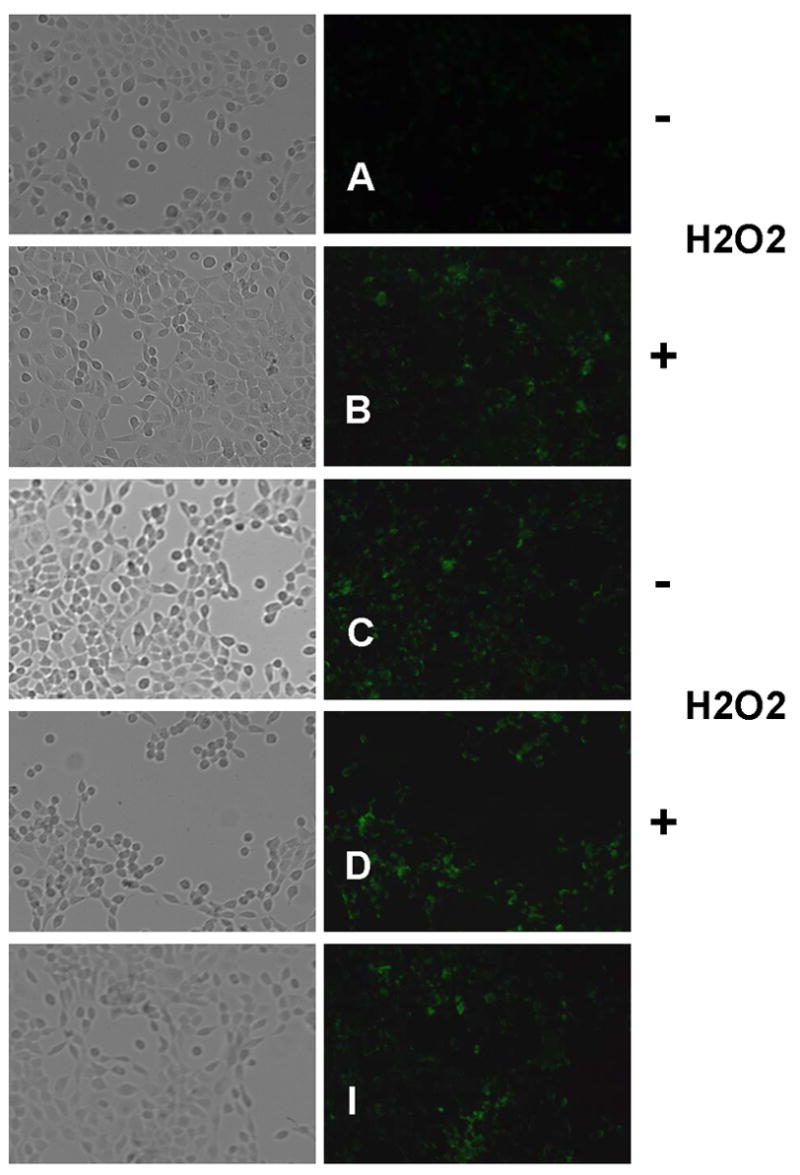

Anti-Apoptotic Effects of ECG and EGCG on H2O2-treated UROtsa Human Bladder Cells. Cells were treated with 40 μg/mL catechin agent or 1 mM Tiron in the presence or absence of 1 mM H2O2 and analyzed for apoptosis 24 h later using the Annexin V detection kit as described in Materials and methods. Annexin V-labeled apoptotic cells (green fluorescence) within the same microscopic field were viewed and imaged using bright field and fluorescence settings (FITC filter). For quantification, fluorescence was expressed as relative fluorescence units (RFU) per cell. (A, B) Negative control; (C, D) Tiron treatment; (E, F) ECG treatment; (G, H) EGCG treatment; (I) Positive (EtOH) control. Representative images are from three independent experiments.
Catchins Modulate Apoptosis in Normal Human Bladder Cells Via Alteration of Reactive Oxygen Species (ROS) Signaling
To investigate the mechanism of H2O2-induced apoptosis, the effects of catalase (CAT) and superoxide dismutase (SOD) on H2O2-induced cell death in UROtsa cells were investigated. Addition of CAT, a H2O2 scavenger, significantly prevented (p < 0.01, n=8) UROtsa cell apoptosis induced by 1 mM H2O2 (Fig. 5). Conversely, no effect on UROtsa cell death was observed with SOD treatment. Combined enzyme treatment, in the presence of 1 mM H2O2, significantly enhanced (p < 0.01, n=8) UROtsa cell survival (Fig. 5), though the effect was less pronounced than CAT alone. Like SOD, Tiron (1 mM) failed to protect UROtsa cells from H2O2-induced cell death. These findings suggest that the mode of H2O2-induced UROtsa cell death is mediated partly via O2−· and possibly through direct H2O2 mechanisms.
Fig. 5.
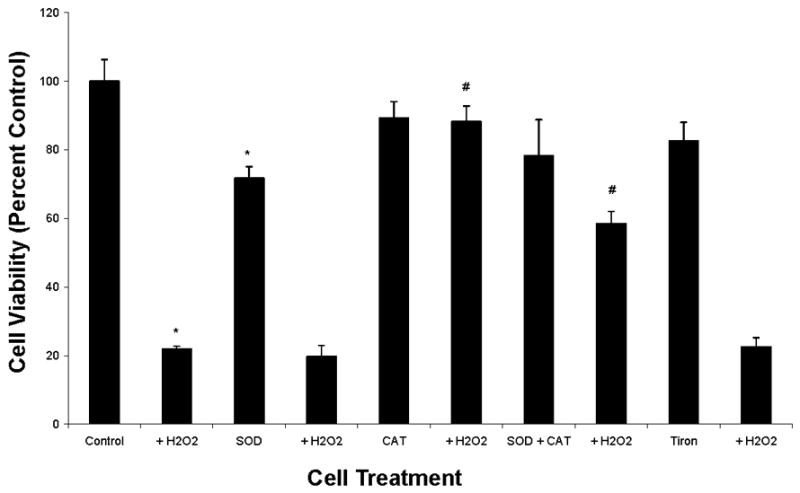
Reactive Oxygen Species (ROS) Production in UROtsa Human Bladder Cells: Effects of Catalase (CAT) and Superoxide Dismutase (SOD) on Cell Viability. Cells were treated with 200 U of CAT, 200 U of SOD, CAT/SOD combination (200 U each) or 1 mM Tiron in the presence or absence of 1 mM H2O2 and XTT assay performed as described in Materials and methods. Data (means ± SEM) are representative of three independent experiments. *Significant difference at p < 0.01 versus control; #Significant difference at p < 0.01 versus H2O2.
H2O2 Stimulates ROS Production in Normal Human Bladder Cells
To further confirm production/mechanistic involvement of O2−· in UROtsa bladder cell death, ROS levels were quantified with coelenterazine cp following acute (5 min) exposure to H2O2. We observed that treatment of UROtsa cells with 1 mM H2O2 caused a significant increase (p < 0.01, n=5–8) in ROS (Fig. 6). Exogenous treatment with Tiron (1 mM) or EGCG (40 μg/mL), in the presence of 1 mM H2O2, significantly reduced ROS generation (p < 0.01, n=5–8), though EGCG was more effective Fig. 6).
Fig. 6.

Reactive Oxygen Species (ROS) Induction by H2O2 in UROtsa Human Bladder Cells: Scavenging effects of EGCG. Cells were treated with 40 μg/mL EGCG or 1 mM Tiron in the presence or absence of 1 mM H2O2 and evalulated for ROS production as described in Materials and methods. Data (means ± SEM) are representative of three independent experiments. *Significant difference at p < 0.01 versus control; #Significant difference at p < 0.01 versus H2O2.
Discussion
In this study we evaluated the anti-oxidant/anti-inflammatory properties of GTE, PP-60, ECG and EGCG in human bladder uroepithelial cells following acute oxidative stress (H2O2). These catechins have demonstrated great potential as anti-carcinogenic agents (Wang et al., 1992; Qin et al., 2007), presumably via anti-oxidant and cell signaling properties. Our results revealed that PP-60, ECG and EGCG differentially protect human bladder cells against H2O2-induced stress/apoptosis mediated partially via superoxide and/or direct H2O2 mechanisms. These findings suggest that dietary intervention with catechins (green tea) may have clinical relevance regarding protection from chemical irritation, neurodegeneration and inflammation of the bladder.
Very little is known about the protective abilities/anti-inflammatory properties of green tea/catechins in cells of the lower urinary tract, especially in bladder urothelium. Two previous studies have focused on the neuroprotective effects of catechins and potential neuro-like signaling capabilities of urothelial cells. Using rat mesencephalic cells, Nobre Júnior et al. (2003) observed (−)-Catechin protection from 6-hydroxydopamine (6-OHDA)-induced apoptosis and lipid peroxidation (marker of oxidative stress), possibly via induction of glutathione-S-transferase activity. Consistent with these findings, our results showed that PP-60, ECG and EGCG can protect human bladder urothelial cells against acute H2O2-induced oxidative damage (Figs. 2–4). The authors (Nobre Júnior et al., 2003) also speculated that (−)-Catechin, via its antioxidant properties, prevents 6-OHDA-induced ONOO− formation. Similarly, in our studies, Tiron (O2−· scavenger) exhibited minimal effects on ROS generation, whereas EGCG significantly suppressed ROS production (Fig. 6), suggesting EGCG (as well as PP-60 and ECG) suppresses ONOO− formation and/or modulates nitrite levels. Previous studies have documented the oxidative effects of H2O2 in vascular cells, demonstrating its use as a short-term stimulus for oxidative stress (Coyle et al., 2006; Li et al., 2001). In a study by Coyle et al. (2006), H2O2 was found to stimulate increased formation of O2−· in endothelial cells but not in bladder cells, suggesting a potential for differential ROS signaling between endothelial and urothelial cells. Further studies are warranted to determine if this difference could provide therapeutic benefits.
Recently, Chaturvedi et al. (2006) demonstrated that black tea extract (BTE) imparts neuroprotection to dopaminergic neurons in a rat Parkinson’s model. The authors found that BTE partially restored CAT and SOD enzyme activities and thus cell viability following 6-OHDA-induced oxidative stress. We observed that exogenous addition of CAT significantly prevented H2O2-induced UROtsa cell apoptosis, though SOD was ineffective (Fig. 5). Whether this cytoprotective affect occurs via upregulation of CAT activity, increased availability of CAT and/or altered CAT gene expression warrants further investigation. Differential effects of catechins, disparate cell types (neuronal versus non-neuronal), and/or different inflammatory mechanisms, may explain differences between our findings and those of Chaturvedi et al. (2006).
In a more recent study, Burckhardt and coworkers (2008) observed a reduction in oxidative stress with green tea polyphenol (GTP) treatment in an animal model of intermittent hypoxia. Upregulation of expression of NADPH oxidase, a superoxide producing enzyme, was mediated upon exposure to GTP. These findings suggest a gene signaling role for green tea and its associated catechins that can modulate the expression of inflammatory enzymes (as NADPH oxidase is upregulated in disease). Furthermore, these results support our findings that green tea polyphenols can modulate and/or scavenge ROS and may modulate gene expression in bladder cells, though further studies are needed to thoroughly examine green tea’s mechanism of action.
Based upon our findings, apoptotic induction by acute H2O2 exposure in UROtsa cells may be mediated partially by O2−· and/or direct H2O2-based signaling. Although the precise molecular mechanism(s) by which PP-60, ECG and EGCG exert their protection against bladder cell oxidative damage remains to be established, it may be mediated by protein kinase C (PKC) and/or nuclear factor-kappa beta (NF-κB) signaling. Preliminary experiments examining gene upregulation (data not shown) suggest that NF-κB linked gene expression is modulated (potentially downregulated) with exposure to EGCG in UROtsa cells, further supporting this potential mechanism. However, additional detailed experiments are needed to confirm this hypothesis. Indeed, PKC and NF-κB are well known for their roles in cell survival, programmed cell death, ROS signaling/modulation and inflammation. In neuronal cells, EGCG has been suggested to induce PKC activity, resulting in neuroprotection (Mandel and Youdin, 2004). Also, NF-κB inhibition has been shown to suppress oxidative stress-induced epithelial cell cytotoxicity/death (Jin et al., 2007; Syed et al., 2007). We speculate that similar signaling mechanisms mediate PP-60, ECG and EGCG bladder cytoprotection, and further studies are underway to probe changes in gene expression with catechin treatment.
Overall, our results demonstrate that the catechins PP-60, ECG and EGCG protect against bladder urothelial cell death, suggesting they can overcome oxidative stress. Because tea polyphenol studies in bladder are scarce, further basic mechanistic studies regarding catechin usage in bladder inflammation/disease would prove clinically relevant. Indeed, catechins are known to downregulate ROS formation and suppress neurogenic inflammation in hyperactive bladder (Chen et al., 2004). These effects may translate into dietary treatment for bladder ailments including interstitial cystitis, a chronic inflammatory condition of the lower urinary tract, in which urothelial cell damage has been proposed as an important etiology (Chancellor and Yoshimura, 2004). Further detailed studies with green tea, however, are needed that could lead to novel preventative and therapeutic strategies for bladder dysfunction.
Acknowledgments
This work was supported in part via NIH T32 Research Training Awards (T32 DK007774-07) [C.H.C. and B.J.P] and the Fishbein Family Foundation (CURE-IC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Dreosti IE. Bioactive ingredients: antioxidants and polyphenols in tea. Nutrition Reviews. 1996;54:S51–S58. doi: 10.1111/j.1753-4887.1996.tb03819.x. [DOI] [PubMed] [Google Scholar]
- Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Research. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radical Research. 1995;22:375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS Letters. 2000;486:10–13. doi: 10.1016/s0014-5793(00)02197-9. [DOI] [PubMed] [Google Scholar]
- Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Molecular Cancer Research. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Negri E, D’Avanzo B, Savoldelli R, Franceschi S. Genital and urinary tract diseases and bladder cancer. Cancer Research. 1991;51:629–631. [PubMed] [Google Scholar]
- Hofseth LJ, Dunn BP, Rosin MP. Micronucleus frequencies in urothelial cells of catheterized patients with chronic bladder inflammation. Mutation Research. 1996;352:65–72. doi: 10.1016/0027-5107(95)00252-9. [DOI] [PubMed] [Google Scholar]
- Chan MMY, Fong D, Ho CT, Huang HI. Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechin gallate, a natural product from green tea. Biochemical Pharmacology. 1997;54:1281–1286. doi: 10.1016/s0006-2952(97)00504-2. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Matsui MS, Elmets CA, Mukhtar H. Polyphenolic antioxidant (−)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochemistry and Photobiology. 1999;69:148–153. [PubMed] [Google Scholar]
- Yang CS, Lee MJ, Chen L. Human salivary tea catechin levels and catechin esterase activities: implication in human cancer prevention studies. Cancer Epidemiology, Biomarkers & Prevention. 1999;8:83–89. [PubMed] [Google Scholar]
- Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiology, Biomarkers & Prevention. 2002;11:1025–1032. [PubMed] [Google Scholar]
- Duerrschmidt N, Stielow C, Muller G, Pagano PJ, Morawietz H. NO-mediated regulation of NAD(P)H oxidase by laminar shear stress in human endothelial cells. Journal of Physiology. 2006;576:557–567. doi: 10.1113/jphysiol.2006.111070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle CH, Martinez LJ, Coleman MC, Spitz DR, Weintraub NL, Kader KN. Mechanisms of H2O2-induced oxidative stress in endothelial cells. Free Radical Biology & Medicine. 2006;40:2206–2213. doi: 10.1016/j.freeradbiomed.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Li WG, Miller FJ, Jr, Zhang HJ, Spitz DR, Oberley LW, Weintraub NL. H(2)O(2)-induced O(2) production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. Journal of Biological Chemistry. 2001;276:29251–29256. doi: 10.1074/jbc.M102124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpey MM, White CR, Suarez E, Richardson G, Radi R, Freeman BA. Chemiluminescent detection of oxidants in vascular tissue: Lucigenin but not coelenterazine enhances superoxide formation. Circulation Research. 1999;84:1203–1211. doi: 10.1161/01.res.84.10.1203. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Hong JY, Huang MT, Reuhl KR, Conney AH, Yang CS. Inhibition of N-nitrosodiethylamine- and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced tumorigenesis in A/J mice by green tea and black tea. Cancer Research. 1992;52:1943–1947. [PubMed] [Google Scholar]
- Qin J, Xie LP, Zheng XY, Wang YB, Bai Y, Shen HF, Li LC, Dahiya R. A component of green tea, (−)-epigallocatechin-3-gallate, promotes apoptosis in T24 human bladder cancer cells via modulation of the PI3K/Akt pathway and Bcl-2 family proteins. Biochemical and Biophysical Research Communications. 2007;354:852–857. doi: 10.1016/j.bbrc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Nobre Júnior HV, Cunha GMA, Maia FD, Oliveira RA, Moraes MO, Rao VSN. Catechin attenuates 6-hydroxydopamine (6-OHDA)-induced cell death in primary cultures of mesencephalic cells. Comparative Biochemistry and Physiology Part C. 2003;136:175–180. doi: 10.1016/s1532-0456(03)00198-4. [DOI] [PubMed] [Google Scholar]
- Chaturvedi RK, Shukla S, Seth K, Chauhan S, Sinha C, Shukla Y, Agrawal AK. Neuroprotective and neurorescue effect of black tea extract in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Neurobiology of Disease. 2006;22:421–434. doi: 10.1016/j.nbd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Burckhardt IC, Gozal D, Dayyat E, Cheng Y, Li RC, Goldbart AD, Row BW. Green tea catechin polyphenols attenuate behavioral and oxidative responses to intermittent hypoxia. American Journal of Respiratory Critical Care and Medicine Epub. 2008 doi: 10.1164/rccm.200701-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel S, Youdim MBH. Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radical Biology & Medicine. 2004;37:304–317. doi: 10.1016/j.freeradbiomed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Jin XH, Ohgami K, Shiratori K, Koyama Y, Yoshida K, Kase S, Ohno S. Inhibition of nuclear factor-kappa B activation attenuates hydrogen peroxide-induced cytotoxicity in human lens epithelial cells. British Journal of Ophthalmology. 2007;91:369–371. doi: 10.1136/bjo.2006.107037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed DN, Afaq F, Kweon MH, Hadi N, Bhatia N, Spiegelman VS, Mukhtar H. Green tea polyphenol EGCG suppresses cigarette smoke condensate-induced NF-κB activation in normal human bronchial epithelial cells. Oncogene. 2007;26:673–682. doi: 10.1038/sj.onc.1209829. [DOI] [PubMed] [Google Scholar]
- Chen WC, Hayakawa S, Shimizu K, Chien CT, Lai MK. Catechins prevents substance P-induced hyperactive bladder in rats via the downregulation of ICAM and ROS. Neuroscience Letters. 2004;367:213–217. doi: 10.1016/j.neulet.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Chancellor MB, Yoshimura N. Treatment of interstitial cystitis. Urology. 2004;63:85–92. doi: 10.1016/j.urology.2003.10.034. [DOI] [PubMed] [Google Scholar]


