Abstract
Posttraumatic stress disorder (PTSD) is a prevalent disorder that is associated with poor clinical and health outcomes, and considerable health care utilization and costs. Recent estimates suggest that 5% to 20% of military personnel who serve in current conflicts in Iraq and Afghanistan meet diagnostic criteria for PTSD. Clinically, sleep disturbances are core features of PTSD that are often resistant to first-line treatments, independently contribute to poor daytime functioning, and often require sleep-focused treatments. Physiologically, these observations suggest that PTSD is partially mediated by sleep disruption and its neurobiological correlates that are not adequately addressed by first-line treatments. However, polysomnographic studies have provide limited insights into the neurobiological underpinnings of PTSD during sleep. There is an urgent need to apply state-of-the-science sleep measurement methods to bridge the apparent gap between the clinical significance of sleep disturbances in PTSD and the limited understanding of their neurobiological underpinnings. Here, we propose an integrative review of findings derived from neurobiological models of fear conditioning and fear extinction, PTSD, and sleep-wake regulation suggest that the amygdala and medial prefrontal cortex can directly contribute to sleep disturbances in PTSD. Testable hypotheses regarding the neurobiological underpinnings of PTSD across the sleep-wake cycle are offered.
Keywords: Posttraumatic stress disorder, sleep, arousal, amygdala, medial prefrontal cortex, insomnia, nightmares
Introduction
Posttraumatic Stress Disorder (PTSD) is a clinical syndrome characterized by re-experiencing, avoidance, and hyperarousal reactions that persist for more than one month after exposure to a traumatic event. Violent crimes, including rape and physical assaults, combat exposure, and natural disasters constitute examples of traumatic events that can involve threat to integrity of the self or others and can be accompanied by intense fear, helplessness, or horror (1). Trauma exposure is not a rare event: more than two thirds of the general population is exposed to at least one traumatic event over their lifetime (2). Epidemiological studies indicate that community prevalence estimates of PTSD range from 1% to 10% (2;3), with higher estimates reported in victims of interpersonal violence (20% to 30%) (2–4) and combat veterans (15%–30%) (5). In veterans of the current Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF), Hoge et al. (6) found that 31% of OEF service members and 71% to 86% of OIF service members reported multiple combat experiences, and as many as one out of nine troops returning from Afghanistan, and one out of six troops returning from Iraq endorse clinically significant PTSD symptoms (6). These estimates are likely to rise over time, as indicated in a recent report that indicated that 33.4% of OIF/OEF returnees evaluated at VA Healthcare facilities between 2002 and 2006 met diagnostic criteria for mental disorders, including PTSD (7). PTSD is often a chronic condition, and is associated with enormous health care costs in both military and civilian samples (8). Recommended first-line treatments for PTSD include selective serotonin reuptake inhibitors (SSRIs), and cognitive-behavioral approaches such as exposure-based and cognitive therapy (9;10).
There is growing evidence that sleep disruption that occur following trauma exposure may constitute a specific mechanism involved in the pathophysiology of chronic PTSD and poor clinical outcomes. Subjective and objective sleep disturbances occurring early after trauma exposure, as well as heightened sympathovagal tone during REM sleep, are associated with an increased risk of meeting criteria for PTSD at subsequent assessments conducted up to one year later (11–13). Sleep disturbances are a core feature of PTSD. Nightmares and insomnia are diagnostic symptoms of PTSD (1), and other sleep disturbances such as sleep avoidance, sleep terrors, nocturnal anxiety attacks, simple and complex motor behaviors and vocalizations, acting out dreams, sleep apnea, and periodic leg movement disorders are also frequently reported and observed by PTSD patients (14;15). Additionally, sleep disturbances independently exacerbate daytime symptoms, and contribute to poor clinical outcomes in PTSD, such increased severity of depression (16), suicidality (16), and general psychiatric distress (17), poorer quality of life and functioning (17), and poorer perceived physical health (18), and increased alcohol and drug use (19;20). While these associations between sleep disturbances and poor clinical outcomes are derived from a posteriori observations, they stress the need for prospectively monitoring the possible development sleep disturbances in trauma exposed individuals, and the role of sleep disturbances as mediators of the relationship between PTSD and clinical outcomes. Finally, sleep disturbances are often resistant to recommended first-line interventions (21;22). Adjunctive sleep-focused pharmacological or behavioral interventions are commonly used to alleviate PTSD-related nightmares and insomnia. Of note, the use benzodiazepines remain highly common in PTSD, possibly for alleviating daytime anxiety symptoms and sleep disturbances, despite the absence of evidence supporting their efficacy (25;26). Effective treatments of nightmares and insomnia also associated with improvements in daytime PTSD symptoms, depression, quality of life, and perceived physical health (e.g., 23;24;27 see also (4) for review). Together, these observations raise the possibilities that 1) trauma exposure directly alters sleep-wake regulation mechanisms, 2) PTSD is partially mediated by sleep-specific mechanisms, and 3) normalization of altered neurobiological mechanisms underlying sleep disturbances in PTSD requires targeted treatments.
The overarching goal of this paper is integrate convergent lines of evidence derived from sleep neuroimaging studies in related disorders, from waking neuroimaging studies conducted PTSD patients, and from animal models of fear conditioning to provide a preliminary model and testable hypotheses of the neurobiological underpinnings of PTSD during rapid-eye movement (REM) and non-REM (NREM) sleep. Prior sleep findings in PTSD samples are only briefly reviewed here. Extensive critical review of prior qualitative and polysomnographic studies of sleep in PTSD samples and review of pharmacological and behavioral treatments that target PTSD-related sleep disturbances are available elsewhere (28–30). Findings derived from sleep neuroimaging studies in healthy human subjects are then briefly reviewed. Because the hyperactivity of the amygdala and impaired function of the medial frontal cortex are neurobiological correlates of PTSD, results from animal and human studies suggesting that the amygdala and medial prefrontal cortex directly influence the regulation and/or expression of REM and NREM sleep are highlighted. The neurobiology of fear conditioning and fear extinction, complementary animal models of PTSD in humans, their effects on sleep, as well as neuroimaging findings observed in PTSD samples are also presented. Findings from these areas of research evidence potentially significant dual roles of the amygdala and medial prefrontal cortex as both critical structures involved in the fear response and PTSD, and important modulator of NREM and REM sleep. Based on this observation, preliminary models and hypotheses regarding potential neurobiological correlates of PTSD during NREM and REM sleep are described.
An in-depth understanding of the sleep-specific underpinnings of PTSD, acquired with state-of-the-science measurement methods, is essential to guide for development, refinement, and testing of innovative prevention and interventions strategies across the sleep-wake cycle. More broadly, better empirically-derived models of PTSD during sleep may generate novel insights into the pathophysiology, prevention, and treatment of other adjustment and stress-related disorders, such as those affecting cohorts of combat-exposed military veterans, as well as of victims of violent crimes and terrorist attacks, and survivors of natural disasters. Finally, elucidating the neurobiological underpinnings of PTSD during sleep can inform efforts to identify the mechanisms subserving resistance of sleep disturbances to first-line treatments of PTSD, as well as the distinct mechanisms underlying treatment response to sleep treatments in PTSD, and other stress-related disorders.
Sleep neuroimaging findings in healthy human subjects
Consistent with animal models of sleep regulation, sleep neuroimaging studies in healthy humans indicate that specific patterns of neuronal activation and deactivation characterize NREM and REM sleep relative to wakefulness. Specifically, whole-brain glucose metabolism and blood flow are reduced by 30–40% during NREM sleep in healthy subjects relative to wakefulness (31). NREM sleep is also associated with relative reduced metabolic activity and blood flow in the wake-promoting areas including the pontine and midbrain reticular formation and thalamus, as well as in associative cortices (31;32). A relative increase in neuronal activity in regions involved in the generation and maintenance of sleep, such as the dorsal pontine tegmentum and basal forebrain has also been observed (31). Some studies have reported reduced activity of paralimbic cortices, including the anterior cingulate gyrus and parahippocampal gyrus during NREM sleep relative to wakefulness, whereas others have not (31). Braun (32) suggested that this disengagement of paralimbic structures and isolation of limbic structures (e.g., amygdala, hippocampus) from other heteromodal cortices may facilitate the restorative function of NREM sleep (31;32). The pattern of neuronal deactivation observed in NREM sleep relative to wakefulness suggests that NREM is an endogenous state of attenuated arousal.
During REM sleep, whole-brain glucose metabolism is increased by 16% relative to NREM sleep, and non-significantly different from wakefulness levels (32). REM sleep is characterized by increased regional cerebral metabolic activity and blood flow in the amygdala and anterior paralimbic areas, and with increased activity in the medial pons and thalamus relative to wakefulness (32–34). Lateral prefrontal cortices, parietal cortices, and primary sensory cortices are further deactivated relative to wakefulness and NREM sleep during REM sleep. These selective activation and deactivation patterns during REM sleep relative to wakefulness in healthy subjects have yielded the hypothesis that dreams may reflect the mental representations of high limbic activations in conjunctions with deactivation of high-order cortical regions (34). The pattern of activation observed during REM sleep suggests that REM sleep is an endogenous state of heightened activity in emotional arousal brain centers.
The amygdala and medial prefrontal cortex as modulators of REM sleep and NREM sleep
It is clear from animal studies that limbic and paralimbic regions are not among the primary regulators of NREM and REM sleep (35–38). However, sleep neuroimaging studies in humans have shown that neuronal activity in amygdala and anterior paralimbic cortices including the medial prefrontal cortex vary across the sleep wake cycle. Although the amygdala and medial prefrontal cortex are not primary brain sites involved in the regulation of sleep per se, growing evidence suggests that both regions are important modulators of NREM and REM sleep.
Neuronal firing in the amygdala varies across the sleep-wake cycle, with higher firing rates during wakefulness and REM sleep compared to NREM sleep. In healthy human subjects, neuronal activity in the amygdala remains unchanged or is slightly reduced during NREM sleep relative to wakefulness (31;32;39), and is considerably increased during REM sleep compared to both NREM sleep and wakefulness. The amygdala shares interconnections with the basal forebrain, hypothalamus, preoptic area of the anterior hypothalamus, brainstem reticular formation, and solitary tract nucleus. It also shares reciprocal connections with the REM-on and REM-off centers. Thus, the amygdala is anatomically positioned to influence sleep via its connection to wakefulness-promoting and sleep-promoting areas. Stimulation of the amygdala during REM sleep increases PGO waves in REM and NREM sleep (40), whereas inactivation of the amygdala with tetrodotoxin decreases sleep latency, and increases slow wave activity during wakefulness, REM sleep, NREM sleep (41;42). Lesions of the amygdala in rhesus monkeys are associated with increased sleep consolidation and total sleep time, and sleep consolidation is proportional to lesion size (43).
The medial prefrontal cortex, and especially the orbitofrontal cortex (OFC), influence sleep, and more specifically NREM sleep. Anatomically, the OFC has afferent and efferent connections with sleep-promoting regions, including the solitary tract nucleus and the ventrolateral preoptic area (VLPOA). Electrical stimulation of the OFC produces EEG synchrony and behavioral sleep, whereas lesions and ablation of the OFC are associated with reduced slow-wave sleep and reductions in behavioral sleep (see (35) for review). Neurons of the subgenual cingulate cortex, another region of the medial frontal cortex, increase their firing rate during NREM sleep in rhesus monkeys (44).
The role of the amygdala and of the medial prefrontal cortex in modulating REM and NREM sleep in humans remains incompletely explored. However, and as described below, functioning and structural abnormalities of the amygdala and medial prefrontal cortex that are suspected to subserve the pathophysiology of PTSD may also directly affect sleep NREM and REM sleep regulation via interconnections between the amygdala, medial frontal cortex, and sleep- and arousal-promoting brain regions.
Neurobiological correlates of fear conditioning, fear extinction, and PTSD
Pavlovian fear conditioning and fear extinction paradigms have been proposed as animal models of PTSD (45). Fear conditioning arises when a neutral stimulus (e.g., light, tone) closely precede in time the occurrence of an aversive, emotionally significant event (e.g., shock) that will elicit a fear response (e.g., freezing). The neutral stimulus is termed the conditioned stimulus (CS), and the aversive event is termed the unconditioned stimulus (UCS). With repetition of the association, the neutral stimulus (CS) alone can elicit the fear response, now termed the conditioned response (CR). With repeated presentation of the CS alone, the conditioned fear response is attenuated and eliminated. This process is called extinction.
During acquisition of fear conditioning, sensory information is transmitted to the lateral amygdala via sensory cortices and thalamus. Information is then transmitted from the lateral amygdala to the central nucleus of the amygdala, which sends projections to hypothalamic and brainstem regions that subserve autonomic and visceral fear responses. Rodent models of fear conditioning, using single-cell and multiunit recordings, c-fos activity, electrical or pharmacological stimulation, lesions, or temporary deactivation methods have shown that the amygdala and medial prefrontal cortex play critical roles in both the acquisition fear conditioning and in fear extinction (see 46 for review). Extinction does not replace or erase the fear CR, but rather, reflects new learning, which competes with the CR. Recall of fear extinction relies heavily on an intact infralimbic cortex in animals (47), which corresponds to the rostral anterior cingulate cortex, medial orbitofrontal cortex, and subcallosal cortex (including the subgenual ACC) in humans. In healthy human subjects, functional neuroimaging studies have confirmed the role of the amygdala in fear conditioning (e.g., 48;49), as well as in fear extinction, and increased activation of the medial prefrontal cortex during fear extinction training and during fear extinction recall (50).
Functional neuroimaging findings in PTSD patients are also consistent with animal models and preclinical studies of fear conditioning and fear extinction in humans. Specifically, waking brain imaging studies indicate that PTSD is characterized by hyper-responsiveness of the amygdala to threat-related stimuli (51–56), and/or blunted responsiveness of the medial prefrontal cortex, which exerts inhibitory control over the amygdala (57;58). Altered perfusion in limbic and frontal regions has also been observed in the absence of trauma reminders. Reduced volume of the anterior cingulate has been reported in PTSD subjects compared to non-PTSD subjects (59;60). Thus, reduced functional activity and reduced volume of the ventromedial prefrontal cortex may both yield reduced inhibition of hyperresponsive amygdala in PTSD. Medication-free PTSD subjects show increased fear conditioning and deficits in fear extinction compared to non-PTSD subjects (61), as well as increased amygdalar activation during fear conditioning, and attenuated activation of the medial prefrontal cortex during extinction compared non-PTSD subjects (62).
Effects of fear conditioning and PTSD on sleep
Animal studies have investigated the acute effects of fear conditioning on sleep as a model of the physiological underpinnings of sleep in PTSD. While this model does not closely reflect the persistence of sleep disturbances long after exposure to the original trauma seen in human PTSD, it nevertheless provide insights into the effects of fear conditioning on sleep and their physiological substrates. In rats and mice, fear conditioning increases REM sleep latency, decrease REM sleep duration (63–65) and number of REM bouts (66), and increases ponto-geniculo-occipital (PGO) waves, a marker of alerting mechanisms during sleep and wakefulness analogous to rapid eye movements in humans (63;65). Alternatively, safety conditioning, where animals learn that they will not be exposed to aversive stimuli in a given environment or given a specific cue never paired with the aversive stimulus, is associated with increased REM sleep duration and percent (66).
The effects of cued fear conditioning on sleep in animals are mediated by amygdala projections to brainstem regions involved in alerting and REM sleep generation (65). In addition, the effects of fear conditioning on sleep appear to be relate to heightened neuronal activity of the brainstem reticular activating system during sleep. In mice, fear conditioning is associated with increased c-fos expression in amygdala, locus coeruleus, and dorsal raphe nucleus, but not in the pontopedunculopontine tegmentum (PPT), and laterodorsal tegmentum (LDT) (67). The sustained and increased in neural activity of amygdala, the locus coeruleus, and dorsal raphe during sleep after fear conditioning disrupt REM sleep, via maintained inhibition of the cholinergic activity responsible for REM sleep generation by increased activity of the LC and dorsal raphe nucleus.
There are no studies on the effects of fear extinction on sleep in animal models. However, the relationship between sleep and fear extinction is highlighted by the effects of sleep deprivation on fear extinction in rats and mice. Specifically, sleep deprivation impairs the acquisition of fear extinction (68). Given that fear extinction relies heavily on an intact medial prefrontal cortex, it seems plausible that sleep deprivation impairs fear extinction via its effects on the prefrontal cortex. In an analogous manner, chronic sleep disruption in PTSD interferes with fear extinction by further impairing or exacerbating impairments of the medial prefrontal cortex.
No study has yet investigated the neurobiological correlates of the effects of fear conditioning or fear extinction on NREM and REM sleep in humans. Multiple polysomnographic studies that compared PTSD and non-PTSD samples have been conducted. Overall, there are discrepancies regarding the presence and nature of objective sleep disturbance. Some PSG studies in PTSD patients have reported REM sleep anomalies e.g., (69–74), whereas other did not e.g. (75–77). NREM sleep anomalies such as reduced slow-wave sleep have also been reported in some (75). A recent meta-analysis found small-medium effect size for increased REM density and increased percentage of stage 1 sleep, and reduced slow wave sleep in PTSD compared to non-PTSD groups (78). It has been hypothesized that REM sleep and NREM sleep mechanisms can underlie the production of posttraumatic nightmares, and contribute to the pathogenesis and maintenance of PTSD (12;72;79). Heightened activity of REM sleep regulation centers and of the amygdala during sleep have also been suggested as neurobiological correlate of REM sleep anomalies in PTSD subjects(79–81). Consistent with Revonsuo’s hypothesis that a function of dreaming is threat simulation and rehearsal of motor patterns involved in escaping threats (82), heightened activation of the amygdala may also subserve the occurrence of PTSD- and non-PTSD related nightmares. (81). However, the neurobiological correlates of REM sleep and NREM sleep in PTSD, as well as the neurobiological correlates of PTSD-related nightmares remain unexplored.
In summary, the amygdala and the medial prefrontal cortex are involved in the neurobiology of PTSD and of the effects of fear conditioning on sleep in animals, in addition to the role they play in modulation of NREM and REM sleep. Heightened amygdala activity, and/or impaired medial prefrontal cortex function observed in PTSD patients may adversely affect the regulation of NREM and REM sleep via their interconnections with arousal- and sleep-promoting brain Both REM sleep and NREM sleep are disrupted in PTSD, but the neurobiology of these sleep disturbances in PTSD have not been elucidated by polysomnographic studies.
Neurobiological hypotheses of PTSD during sleep
The study of the neurobiological correlates of PTSD during NREM and REM sleep offers a unique paradigm to observe natural activation and deactivation patterns in endogenous states of attenuated central arousal and heightened limbic activity, respectively.
To further the prior hypothesis that sleep mechanisms contribute to the pathophysiology of PTSD, we propose that REM sleep amplifies altered function of the amygdala and medial frontal cortex in PTSD patients; amplification of abnormal amygdala activation in combination with reduced activation of the medial prefrontal cortex subserve nightmares. Figure 1a depicts a preliminary model regarding neurobiological correlates of REM sleep in PTSD subjects compared to healthy subjects, and relative to wakefulness. It is first hypothesized that heightened amygdala activity (Figure 1b) and blunted increase in activity of the medial prefrontal cortex (Figure 1c) characterize PTSD subjects compared to non-PTSD healthy subjects during REM sleep. These changes have direct impact on brainstem REM sleep regulation mechanisms, such as increased activity of brainstem REM-off regions (Figure 1d; LC, raphe), and attenuated activity of the brainstem REM-on nuclei (Figure 1e) PPT/LDG). Persistent activity of the LC and raphe and related inhibition of the PPT and LDT would be expected in PTSD subjects, and may directly relate to REM sleep disruption.
Figure 1.
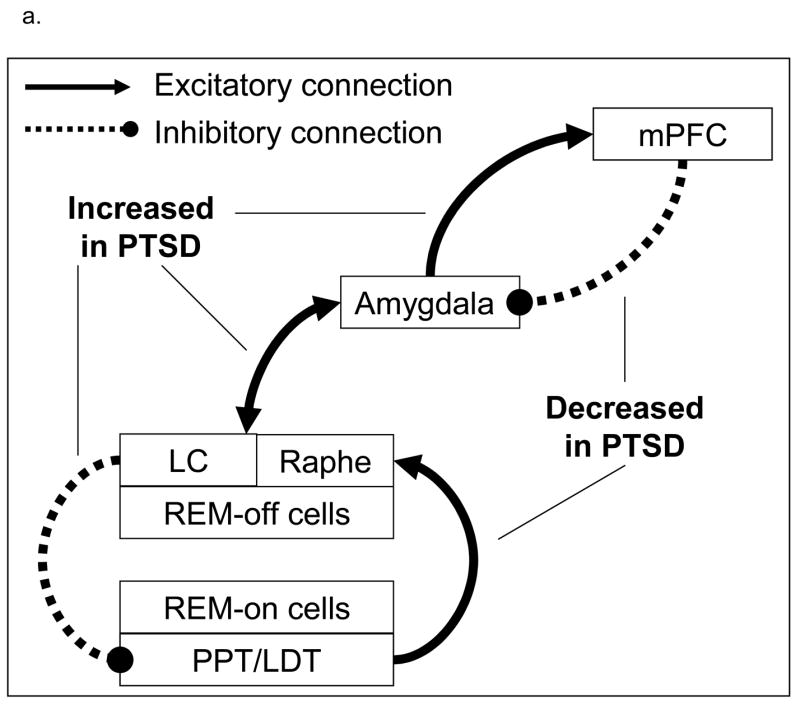
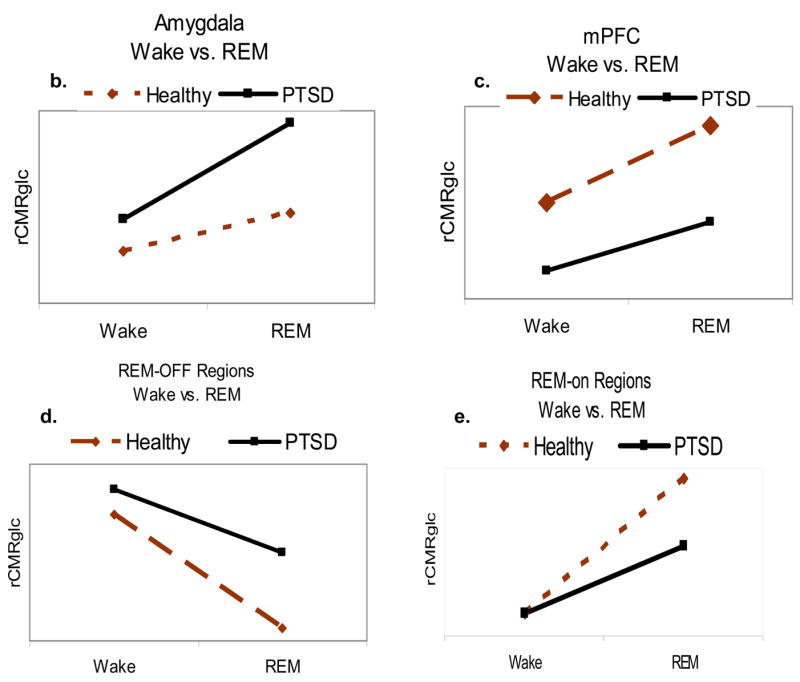
Proposed neurobiological model and hypotheses of PTSD during REM sleep. 1a. Proposed model of PTSD the neurobiological underpinnings of PTSD during REM sleep. 1b through 1e: Relative to wakefulness, PTSD patients (black lines) will show increased activity of the amygdala and brainstem REM-off regions, and decreased activity of the medial prefrontal cortex (mPFC) and of brainstem REM-on regions compared to healthy subjects (dashed lines)
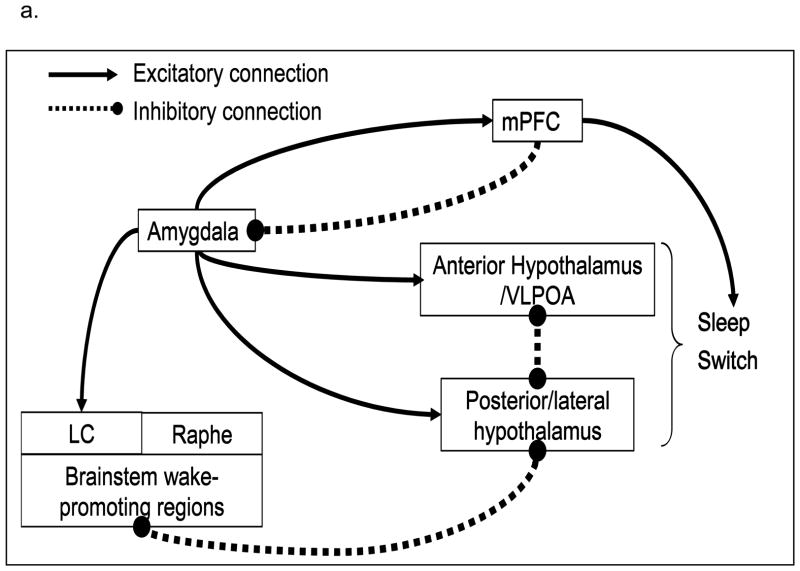
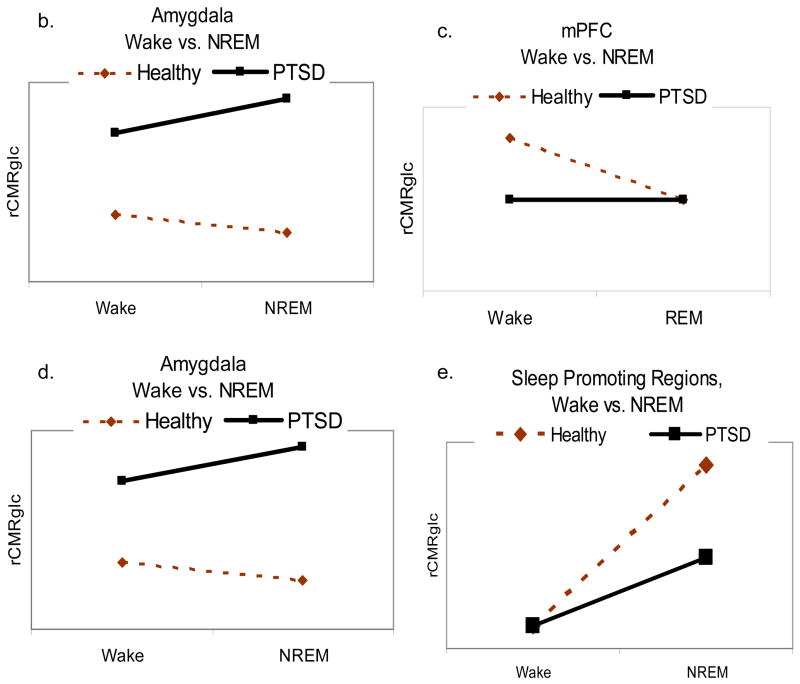
During NREM sleep, we propose that the hyperactivity of the amygdala and attenuated activity of the medial prefrontal cortex contribute to heightened whole-brain neuronal activity. Specifically, these changes may maintain or increase activity in arousal-promoting brain centers, and reduce activity in sleep-promoting centers. The resulting pattern of persistent arousal could directly contribute to complaints of insomnia. Figure 2 depicts the preliminary NREM sleep model and hypotheses regarding neurobiological correlates of NREM sleep relative to wakefulness in PTSD patients compared to healthy subjects. Specifically, it is hypothesized that the relative persistence of amygdala activity (Figure 2b) and blunted activity of the medial prefrontal cortex (Figure 2c) during NREM sleep would be associated with less deactivation of brainstem and forebrain wakefulness-promoting areas (Figure 2d; LC, raphe, posterior hypothalamus, thalamus), and a blunted increase in activation of the anterior hypothalamus, and blunted activation in sleep-promoting regions (Figure 2e), such as the anterior hypothalamus (which includes the VLPOA) and solitary tract nucleus (although it may not be possible to directly observe changes in activity of small or diffuse nuclei given the limits of spatial resolution of current neuroimaging methods).
Figure 2.
Proposed neurobiological correlates of PTSD during NREM sleep. 2a Proposed model of the neurobiological underpinnings of PTSD during NREM sleep. 2b through 2e. Relative to wakefulness, PTSD patients (black lines) will show increased activity of the amygdala and wakefulness-promoting brainstem and forebrain regions, and decreased activity of the medial prefrontal cortex (mPFC) and anterior hypothalamus compared to healthy subjects (dashed lines).
Discussion
PTSD is a prevalent disorder that is often resistant to recommended treatments, and is associated with enormous health care costs. Sleep disturbances are a core feature of PTSD that are often resistant to recommended first-line treatments, and independently contribute to poor clinical outcomes. The contribution of sleep disturbances to long-term health outcomes and costs in PTSD is not currently known, but is likely to be substantial. Emerging evidence suggests that sleep-specific mechanisms underlie the neurobiology of PTSD. However, the neurobiological underpinnings of PTSD as it persists across the sleep-wake cycle remain unexplored using sleep neuroimaging methods.
Sleep research in PTSD samples (as well as in other stress-related disorders such as acute stress disorder, adjustment disorders, prolonged grief disorder) is ripe for the broader use of state-of-the-science sleep neuroimaging methods required to identify the sleep-specific neurobiological underpinnings of PTSD, the correlates of resistance to first-line PTSD treatments, the predictors of response to sleep-focused treatments, and the mechanisms that are normalized by effective sleep treatments.
Future research directions have direct clinical implications in PTSD and sleep research. For instance, little is known on the effects of sleep deprivation and disruption on fear conditioning and fear extinction in healthy human subjects and in patients who stress-related disorders. Understanding the sleep-specific mechanisms that may facilitate fear conditioning and/or impeded fear extinction may be especially important in samples where trauma exposure is a likely event, such as during military deployment, combat exposure, and all emergency responders. Further investigating the role of sleep in the consolidation of traumatic memories as well as in processing emotional and traumatic material also provides an ecologically valid paradigm to further expand cognitive neuroscience models of sleep and memory. More in-depth models of the sleep-specific pathophysiological and neurobiological underpinnings of the relationship between trauma exposure, sleep, and PTSD can also guide the development and refinement of innovative prevention and intervention strategies targeting sleep disturbances in trauma-exposed and PTSD samples. For instance, an in-depth understanding of the sleep-related brain mechanisms susceptible to disruption following trauma exposure and in PTSD may facilitate treatment optimization by combining treatments that restore affected neural networks, and/or that enhance compensatory mechanisms. Identifying sleep-specific markers of vulnerability and resilience to chronic, maladaptive stress response and of sleep-focused treatment response may new venues to prevent PTSD in high-risk samples (e.g., combat veterans, emergency workers). Finally, the nature of sleep-specific predictors of treatment response or failure to first-line PTSD treatments, or the underpinnings of effective sleep-focused pharmacological or behavioral interventions have not yet been explored.
In summary, the study of the neurobiological correlates of PTSD during sleep by using state-of-the-science sleep neuroimaging methods opens multiple opportunities to identify the sleep-specific underpinnings of this pervasive disorder, which in turn can inform the development of evidence-based interventions that normalize the underpinnings of PTSD across the sleep-wake cycle.
Practice Points
Sleep disturbances often develop into independent, comorbid sleep disorders in adults with PTSD.
Complaints of sleep disturbances in adults with PTSD contribute to mental and physical health outcomes, including exacerbation of daytime PTSD symptom severity, anxiety, depression, irritability, cognitive functioning, and disability. Sleep disturbances, and potentially their more distal consequences, can be significant ameliorated with sleep-focused treatment.
A thorough evaluation of the nature and adverse impacts of sleep disturbances on daytime symptoms and overall functioning should be integral to PTSD evaluation.
Sleep disturbances comorbid to PTSD require targeted interventions.
Randomized controlled trials indicate that prazosin and nefazodone can effectively reduce nightmares and insomnia in PTSD. Other pharmacological interventions such as cyproheptadine, trazodone, zaleplon, and zolpidem may also reduce nightmares and insomnia, but formal clinical trials are required to fully assess their efficacy, safety, and durability in military and civilians PTSD samples.
Behavioral interventions for PTSD-related sleep disturbances such as imagery rehearsal, and behavioral insomnia treatments have received most empirical evidence for efficacy to date in both military and civilian PTSD samples.
Research Agenda
In order to further refine our understanding of the pathophysiology of PTSD during sleep and to translate these findings into clinical practice, we need to:
Employ available sleep neuroimaging techniques to identify and probe the pathophysiological and neurobiological underpinnings of PTSD across the sleep-wake cycle;
Investigate the neurophysiological and neurobiological mechanisms that underlie sleep-focused treatment response and resistance in PTSD patients;
Develop and test innovative pharmacological and cognitive-behavioral interventions that specifically target and normalize altered physiological and neurobiological systems that subserve sleep disturbances in PTSD;
Conduct mechanistic, longitudinal studies to assess the independent effects of sleep disturbances on health outcomes in PTSD.
Acknowledgments
This manuscript was support by the Department of Defense Peer Reviewed Medical Research Program (PR054093), and the National Institutes of Health (MH60473; MH24652; MH061566; MH066227).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 4.Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61(6):984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- 5.Weiss DS, Marmar CR, Schlenger WE, Fairbank JA, Jordan BK, Hough RL, et al. The prevalence of lifetime and partial post-traumatic stress disorder in Vietnam theater veterans. J Trauma Stress. 1992;5:365–376. [Google Scholar]
- 6.Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 7.Kang HK. www iom edu. 2006. VA health care utilization among Operation Iraqi Freedom/Operation Endurieng Freedom veterans. [Google Scholar]
- 8.Walker EA, Katon W, Russo J, Ciechanowski P, Newman E, Wagner AW. Health care costs associated with posttraumatic stress disorder symptoms in women. Arch Gen Psychiatry. 2003;60(4):369–374. doi: 10.1001/archpsyc.60.4.369. [DOI] [PubMed] [Google Scholar]
- 9.Ballenger JC, Davidson JR, Lecrubier Y, Nutt DJ, Foa EB, Kessler RC, et al. Consensus statement on posttraumatic stress disorder from the International Consensus Group on Depression and Anxiety. J Clin Psychiatry. 2000;61(Suppl 5):60–66. [PubMed] [Google Scholar]
- 10.The expert consensus guideline series. Treatment of Posttraumatic Stress Disorder. The Expert Consensus Panels for PTSD. J Clin Psychiatry. 1999;60(Suppl 16):3–76. [PubMed] [Google Scholar]
- 11.Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159(5):855–857. doi: 10.1176/appi.ajp.159.5.855. [DOI] [PubMed] [Google Scholar]
- 12.Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159(10):1696–1701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- 13.Mellman TA, Knorr BR, Pigeon WR, Leiter JC, Akay M. Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biol Psychiatry. 2004;55(9):953–956. doi: 10.1016/j.biopsych.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Germain A, Hall M, Krakow B, Shear MK, Buysse DJ. A brief sleep scale for posttraumatic stress disorder: Pittsburgh Sleep Quality Index Addendum for PTSD. J Anxiety Disord. 2005;19(2):233–244. doi: 10.1016/j.janxdis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Krakow B, Melendrez D, Pedersen B, Johnston L, Hollifield M, Germain A, et al. Complex insomnia: insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry. 2001;49(11):948–953. doi: 10.1016/s0006-3223(00)01087-8. [DOI] [PubMed] [Google Scholar]
- 16.Krakow B, Artar A, Warner TD, Melendrez D, Johnston L, Hollifield M, et al. Sleep disorder, depression, and suicidality in female sexual assault survivors. Crisis. 2000;21(4):163–170. doi: 10.1027//0227-5910.21.4.163. [DOI] [PubMed] [Google Scholar]
- 17.Krakow B, Melendrez D, Johnston L, Warner TD, Clark JO, Pacheco M, et al. Sleep-disordered breathing, psychiatric distress, and quality of life impairment in sexual assault survivors. J Nerv Ment Dis. 2002;190(7):442–452. doi: 10.1097/00005053-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Clum GA, Nishith P, Resick PA. Trauma-related sleep disturbance and self-reported physical health symptoms in treatment-seeking female rape victims. J Nerv Ment Dis. 2001;189(9):618–622. doi: 10.1097/00005053-200109000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saladin ME, Brady KT, Dansky BS, Kilpatrick DG. Understanding comorbidity between PTSD and substance use disorders: two preliminary investigations. Addict Behav. 1995;20(5):643–655. doi: 10.1016/0306-4603(95)00024-7. [DOI] [PubMed] [Google Scholar]
- 20.Nishith P, Resick PA, Mueser KT. Sleep difficulties and alcohol use motives in female rape victims with posttraumatic stress disorder. J Trauma Stress. 2001;14(3):469–479. doi: 10.1023/A:1011152405048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zayfert C, DeViva JC. Residual insomnia following cognitive behavioral therapy for PTSD. J Trauma Stress. 2004;17(1):69–73. doi: 10.1023/B:JOTS.0000014679.31799.e7. [DOI] [PubMed] [Google Scholar]
- 22.Davidson JR, Rothbaum BO, van der Kolk BA, Sikes CR, Farfel GM. Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry. 2001;58(5):485–492. doi: 10.1001/archpsyc.58.5.485. [DOI] [PubMed] [Google Scholar]
- 23.Krakow B, Hollifield M, Johnston L, Koss M, Schrader R, Warner TD, et al. Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: a randomized controlled trial. JAMA. 2001;286(5):537–545. doi: 10.1001/jama.286.5.537. [DOI] [PubMed] [Google Scholar]
- 24.Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 25.Mellman TA, Clark RE, Peacock WJ. Prescribing patterns for patients with posttraumatic stress disorder. Psychiatr Serv. 2003;54(12):1618–1621. doi: 10.1176/appi.ps.54.12.1618. [DOI] [PubMed] [Google Scholar]
- 26.Cates ME, Bishop MH, Davis LL, Lowe JS, Woolley TW. Clonazepam for treatment of sleep disturbances associated with combat-related posttraumatic stress disorder. Ann Pharmacother. 2004;38(9):1395–1399. doi: 10.1345/aph.1E043. [DOI] [PubMed] [Google Scholar]
- 27.Germain A, Shear MK, Hall M, Buysse DJ. Effects of a brief behavioral treatment for PTSD-related sleep disturbances: A pilot study. Behav Res Ther. 2007;45:627–632. doi: 10.1016/j.brat.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567–590. doi: 10.2165/00023210-200620070-00003. [DOI] [PubMed] [Google Scholar]
- 29.Harvey AG, Jones C, Schmidt DA. Sleep and posttraumatic stress disorder: a review. Clin Psychol Rev. 2003;23(3):377–407. doi: 10.1016/s0272-7358(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 30.Pillar G, Malhotra A, Lavie P. Post-traumatic stress disorder and sleep-what a nightmare! Sleep Med Rev. 2000;4(2):183–200. doi: 10.1053/smrv.1999.0095. [DOI] [PubMed] [Google Scholar]
- 31.Nofzinger EA, Buysse DJ, Miewald JM, Meltzer CC, Price JC, Sembrat RC, et al. Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain. 2002;125:1105–1115. doi: 10.1093/brain/awf103. [DOI] [PubMed] [Google Scholar]
- 32.Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 33.Nofzinger EA, Mintun MA, Wiseman MB, Kupfer DJ, Moore RY. Forebrain activation in REM sleep: An FDG PET study. Brain Res. 1997;770:192–201. doi: 10.1016/s0006-8993(97)00807-x. [DOI] [PubMed] [Google Scholar]
- 34.Maquet P, Peters J, Aerts J, Delfiore G, Degueldre C, Luxen A, et al. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383(6596):163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- 35.Jones BE. Basic mechanisms of sleep-wake states. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 2005. pp. 136–153. [Google Scholar]
- 36.Sinton CM, McCarley RW. Neuroanatomical and neurophysiological aspects of sleep: basic science and clinical relevance. Semin Clin Neuropsychiatry. 2000;5(1):6–19. doi: 10.153/SCNP00500006. [DOI] [PubMed] [Google Scholar]
- 37.Siegel JM. Mechanisms of sleep control. J Clin Neurophysiol. 1990;7(1):49–65. doi: 10.1097/00004691-199001000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szymusiak R, Alam N, McGinty D. Discharge patterns of neurons in cholinergic regions of the basal forebrain during waking and sleep. Behav Brain Res. 2000;115(2):171–182. doi: 10.1016/s0166-4328(00)00257-6. [DOI] [PubMed] [Google Scholar]
- 39.Maquet P, Degueldre C, Delfiore G, Aerts J, Peters JM, Luxen A, et al. Functional neuroanatomy of human slow wave sleep. J Neurosci. 1997;17(8):2807–2812. doi: 10.1523/JNEUROSCI.17-08-02807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deboer T, Sanford LD, Ross RJ, Morrison AR. Effects of electrical stimulation in the amygdala on ponto-geniculo-occipital waves in rats. Brain Res. 1998;793(1–2):305–310. doi: 10.1016/s0006-8993(98)00178-4. [DOI] [PubMed] [Google Scholar]
- 41.Sanford LD, Yang L, Liu X, Tang X. Effects of tetrodotoxin (TTX) inactivation of the central nucleus of the amygdala (CNA) on dark period sleep and activity. Brain Res. 2006;1084(1):80–88. doi: 10.1016/j.brainres.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 42.Tang X, Yang L, Liu X, Sanford LD. Influence of tetrodotoxin inactivation of the central nucleus of the amygdala on sleep and arousal. Sleep. 2005;28(8):923–930. doi: 10.1093/sleep/28.8.923. [DOI] [PubMed] [Google Scholar]
- 43.Benca RM, Obermeyer WH, Shelton SE, Droster J, Kalin NH. Effects of amygdala lesions on sleep in rhesus monkeys. Brain Res. 2000;879(1–2):130–138. doi: 10.1016/s0006-8993(00)02761-x. [DOI] [PubMed] [Google Scholar]
- 44.Rolls ET, Inoue K, Browning A. Activity of primate subgenual cingulate cortex neurons is related to sleep. J Neurophysiol. 2003;90(1):134–142. doi: 10.1152/jn.00770.2002. [DOI] [PubMed] [Google Scholar]
- 45.Grillon C, Southwick SM, Charney DS. The psychobiological basis of posttraumatic stress disorder. Mol Psychiatry. 1996;1(4):278–297. [PubMed] [Google Scholar]
- 46.Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30(2):188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 48.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20(5):937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 49.Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: stimulus processing versus response expression. Behav Neurosci. 2003;117(1):3–10. doi: 10.1037//0735-7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- 50.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 51.Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52(4):305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- 52.Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001;158(11):1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 53.Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, et al. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol Psychiatry. 2003;53(3):204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- 54.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 55.Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996;53(5):380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 56.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 57.Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45(7):806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 59.Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S. Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry. 2006;59(7):582–587. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 60.Kitayama N, Quinn S, Bremner JD. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J Affect Disord. 2006;90(2–3):171–174. doi: 10.1016/j.jad.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109(2):290–298. [PubMed] [Google Scholar]
- 62.Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35(6):791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jha SK, Brennan FX, Pawlyk AC, Ross RJ, Morrison AR. REM sleep: a sensitive index of fear conditioning in rats. Eur J Neurosci. 2005;21(4):1077–1080. doi: 10.1111/j.1460-9568.2005.03920.x. [DOI] [PubMed] [Google Scholar]
- 64.Sanford LD, Yang L, Tang X. Influence of contextual fear on sleep in mice: a strain comparison. Sleep. 2003;26(5):527–540. doi: 10.1093/sleep/26.5.527. [DOI] [PubMed] [Google Scholar]
- 65.Sanford LD, Silvestri AJ, Ross RJ, Morrison AR. Influence of fear conditioning on elicited ponto-geniculo-occipital waves and rapid eye movement sleep. Arch Ital Biol. 2001;139(3):169–183. [PubMed] [Google Scholar]
- 66.Pawlyk AC, Jha SK, Brennan FX, Morrison AR, Ross RJ. A rodent model of sleep disturbances in posttraumatic stress disorder: the role of context after fear conditioning. Biol Psychiatry. 2005;57(3):268–277. doi: 10.1016/j.biopsych.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 67.Liu X, Tang X, Sanford LD. Fear-conditioned suppression of REM sleep: relationship to Fos expression patterns in limbic and brainstem regions in BALB/cJ mice. Brain Res. 2003;991(1–2):1–17. doi: 10.1016/j.brainres.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Silvestri AJ. REM sleep deprivation affects extinction of cued but not contextual fear conditioning. Physiol Behav. 2005;84(3):343–349. doi: 10.1016/j.physbeh.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 69.Ross RJ, Ball WA, Dinges DF, Kribbs NB, Morrison AR, Silver SM, et al. Motor dysfunction during sleep in posttraumatic stress disorder. Sleep. 1994;17(8):723–732. doi: 10.1093/sleep/17.8.723. [DOI] [PubMed] [Google Scholar]
- 70.Riemann D, Hohagen F, Konig A, Schwarz B, Gomille J, Voderholzer U, et al. Advanced vs. normal sleep timing: effects on depressed mood after response to sleep deprivation in patients with a major depressive disorder. J Affect Disord. 1996;37:121–128. doi: 10.1016/0165-0327(95)00082-8. [DOI] [PubMed] [Google Scholar]
- 71.Mellman TA, Nolan B, Hebding J, Kulick-Bell R, Dominguez R. A polysomnographic comparison of veterans with combat-related PTSD, depressed men, and non-ill controls. Sleep. 1997;20(1):46–51. doi: 10.1093/sleep/20.1.46. [DOI] [PubMed] [Google Scholar]
- 72.Mellman TA, Kumar A, Kulick-Bell R, Kumar M, Nolan B. Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psychiatry. 1995;38(3):174–179. doi: 10.1016/0006-3223(94)00238-X. [DOI] [PubMed] [Google Scholar]
- 73.Greenberg R, Pearlman CA, Gampel D. War neuroses and the adaptive function of REM sleep. Br J Med Psychol. 1972;45(1):27–33. doi: 10.1111/j.2044-8341.1972.tb01416.x. [DOI] [PubMed] [Google Scholar]
- 74.Engdahl BE, Eberly RE, Hurwitz TD, Mahowald MW, Blake J. Sleep in a community sample of elderly war veterans with and without posttraumatic stress disorder. Biol Psychiatry. 2000;47(6):520–525. doi: 10.1016/s0006-3223(99)00201-2. [DOI] [PubMed] [Google Scholar]
- 75.Woodward SH, Bliwise DL, Friedman MJ, Gusman DF. Subjective versus objective sleep in Vietnam combat veterans hospitalized for PTSD. J Trauma Stress. 1996;9(1):137–143. doi: 10.1007/BF02116839. [DOI] [PubMed] [Google Scholar]
- 76.Hurwitz TD, Mahowald MW, Kuskowski M, Engdahl BE. Polysomnographic sleep is not clinically impaired in Vietnam combat veterans with chronic posttraumatic stress disorder. Biol Psychiatry. 1998;44(10):1066–1073. doi: 10.1016/s0006-3223(98)00089-4. [DOI] [PubMed] [Google Scholar]
- 77.Lavie P, Katz N, Pillar G, Zinger Y. Elevated awaking thresholds during sleep: characteristics of chronic war-related posttraumatic stress disorder patients. Biol Psychiatry. 1998;44(10):1060–1065. doi: 10.1016/s0006-3223(98)00037-7. [DOI] [PubMed] [Google Scholar]
- 78.Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: A meta-analytic review. Psychophysiology. 2007;44(4):660–669. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- 79.Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of post-traumatic stress disorder. Am J Psychiatry. 1989;146(6):697–707. doi: 10.1176/ajp.146.6.697. [DOI] [PubMed] [Google Scholar]
- 80.Woodward SH, Leskin GA, Sheikh JI. Movement during sleep: associations with posttraumatic stress disorder, nightmares, and comorbid panic disorder. Sleep. 2002;25(6):681–688. [PubMed] [Google Scholar]
- 81.Levin R, Nielsen TA. Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychol Bull. 2007;133(3):482–528. doi: 10.1037/0033-2909.133.3.482. [DOI] [PubMed] [Google Scholar]
- 82.Revonsuo A. The reinterpretation of dreams: an evolutionary hypothesis of the function of dreaming. Behav Brain Sci. 2000;23(6):877–901. doi: 10.1017/s0140525x00004015. [DOI] [PubMed] [Google Scholar]