Abstract
Drug delivery research employing micelles and nanoparticles has expanded in recent years. Of particular interest is the use of these nanovehicles that deliver high concentrations of cytotoxic drugs to diseased tissues selectively, thus reducing the agent’s side effects on the rest of the body. Ultrasound, traditionally used in diagnostic medicine, is finding a place in drug delivery in connection with these nanoparticles. In addition to their non-invasive nature and the fact that they can be focused on targeted tissues, acoustic waves have been credited with releasing pharmacological agents from nanocarriers, as well as rendering cell membranes more permeable. In this article, we summarize new technologies that combine the use of nanoparticles with acoustic power both in drug and gene delivery.
Ultrasonic drug delivery from micelles usually employs polyether block copolymers, and has been found effective in vivo for treating tumors. Ultrasound releases drug from micelles, most probably via shear stress and shock waves from collapse of cavitation bubbles. Liquid emulsions and solid nanoparticles are used with ultrasound to deliver genes in vitro and in vivo. The small packaging allows nanoparticles to extravasate into tumor tissues. Ultrasonic drug and gene delivery from nano-carriers has tremendous potential because of the wide variety of drugs and genes that could be delivered to targeted tissues by fairly non-invasive means.
Keywords: targeted delivery, polymeric micelles, thermo-responsive polymers, ultrasound, non viral gene transfection, drug delivery, nanoemulsions, solid nanoparticles, liposomes
1. Introduction
Nanotechnology has finally and firmly entered the realm of drug delivery. Such is an obvious match – to combine the fields of medicine and therapeutic delivery with the up-and-coming universe of nanotechnology and nanoparticles. Although the cells themselves are larger than the usual size that demarks a nanoparticle, the targets of therapeutic drugs – the membrane protein complexes, membrane pores, organelles, ribosomes, chromosomes, and even DNA itself – are nanosized structures. Thus it is natural and expected that as the technology for nanoparticle manipulation and nanoscale visualization has matured, so has the technology to manipulate biology and drug carriers on the nanoscale to produce better health and life for mankind.
Time and space do not permit us to review all of the nanotechnology innovations that have been introduced into medicine during the past two decades. Instead the focus will be on the use of ultrasound and nanosized drug carriers to deliver drugs, genes and other therapeutic agents specifically to their targeted sites in the body. Targeted drug delivery is essential to modern medicine in which specifically designed and effective drugs are employed to work on selected tissues, cells, and cellular structures. For example, in chemotherapy one often would like to deliver a fairly toxic chemotherapeutic agent directly to a target tissue or location instead of injecting it systemically into the whole body. Similarly in gene therapy for cardiac or brain tissues, it is essential to deliver the gene to that organ only, and perhaps to even a small volume within that organ. Not only does localized delivery use less of the often very costly drug or gene, but also such localized delivery spares the rest of the body from exposure to the therapeutic, resulting in fewer of the detrimental side effects that often accompany drug and gene therapy. In this review the main modality of localized drug delivery is the use of focused ultrasound to effect drug or gene release at the target tissue, and to stimulate the targeted cells in a manner to render them more prone to drug or 5 gene uptake, or more susceptible to therapeutic processes. This review presents those technologies that use ultrasound in combination with nanoscale therapeutics or therapeutic carriers such as micelles and other nanoparticles.
1.1 Ultrasound
Ultrasound (US) consists of pressure waves having frequencies of 20 kHz or greater. Most often the US is generated by piezoelectric transducers that change an applied voltage into mechanical displacement of a surface (the face of the transducer) that is in contact with water, gel, or some other media that can efficiently transmit ultrasonic waves. Usually the transducers are designed to couple the sound waves into body tissue, but not into air. Thus, the transducer must be placed in direct contact with tissue or skin, and the air in between must be excluded through the application of a fluid such as water or ultrasonic gel. Such a simple application has obvious advantages in that there is no surgery or other invasive procedures, thus eliminating pain and minimizing patient aversion to such therapies.
Like optical and audio waves, ultrasonic waves can be focused, reflected and refracted through a medium [1–4]. Thus US can be carefully controlled and focused on the tumor site or a particular tissue volume within the body. As mentioned, such site-specific treatment is beneficial in drug delivery to localize the drug interactions to the target tissue only, thus sparing the body from deleterious side-effects.
Although often compared to light waves, US is a very physical phenomenon; the pressure waves constituting US actually cause a compression and expansion of the transmitting medium, and there is a slight oscillatory displacement of the medium as the pressure waves pass through. This movement creates forces that physically push and stress cells and tissues, but not with sufficient force to disrupt cell membranes, unless gas bubbles are present [5–7].
Another difference between ultrasonic sound waves and light waves is the amount of absorption or scattering that occurs as the wave passes through the medium. Whereas visible light can only penetrate very short distances in tissue (except near infrared which has less absorption), ultrasound can penetrate fairly deeply, depending upon the wavelength and the tissue type. Water and gel have very little absorption and scattering (collectively called attenuation), but muscle has fairly high attenuation, and bone and lung have very high attenuation. This means that ultrasound can “penetrate” into tissues better than can light, but it does not penetrate easily into or through bone or lung. In general the amount of attenuation increases as the frequency of the US increases, so low frequency US can penetrate more deeply into tissues. The acoustic parameters of various tissues at US frequencies have been collected and published elsewhere [8, 9].
There are other significant advantages that render US useful in drug delivery. Some studies have shown a synergistic effect between the pharmacological activity of some drugs and ultrasound [10–15]. Additionally, US enhances drug transport through tissues and across cell membranes by various mechanisms [7, 16–18]. Finally, the absorption of US can be used to create local tissue hyperthermia, which is often employed by itself or as an adjuvant to chemotherapy in treating some tumors [13, 19]. Reviews of “non-nanoparticle” applications of ultrasound in drug delivery can be found elsewhere [20–25].
The interactions between ultrasound and biological tissues are divided into two broad categories: thermal and non-thermal effects. Thermal effects are associated with the absorption of acoustic energy by the fluids or tissues [5]. Non-thermal bioeffects are generally associated with oscillating or cavitating bubbles, but also include non-cavitating effects such as radiation pressure, radiation torque, and acoustic streaming. With respect to drug delivery, these latter bio-effects are probably not greatly involved except to the degree that the fluid or particle motion (via acoustic streaming or radiation pressure) increases convection currents, thus increasing the transport of drug toward or into the cell.
Cavitation is defined as the oscillation of bubbles in an acoustic field. Cavitation can produce strong stresses on cells, leading to various “bioeffects” which may increase drug interaction by upregulating pathways of various types of stress response, or by physically shearing the cell membrane to allow direct passage of therapeutics into the cytosol. Ultrasound has the ability to excite a wide range of bubble sizes, but the bubbles that can achieve the highest level of oscillation are those whose natural resonant frequencies are near the applied ultrasonic frequency. At relatively low acoustic amplitude, bubbles oscillate at the same frequency as the applied sound waves, and with relatively small expansion and contraction in size. During this mild cavitation, called stable or non-inertial cavitation, the bubbles accumulate dissolved gas from the surrounding liquid and slowly grow in size. As the acoustic pressure increases or as the size of the bubble approaches the resonance size, the oscillations increase in amplitude, become non-linear, and eventually result in the total collapse of the bubble. Collapse occurs as the inertia of the inward-moving water surface overcomes the internal pressure to create supercritical fluid within the bubble. This collapse event, known as inertial or collapse cavitation, creates a shock wave, and generates extremely high pressure and temperature (several thousands of degrees K). Literature reports indicate that these strong forces are capable of causing substantial damage to cells. However, it is important to mention here that non-inertial as well as inertial cavitation can cause damage to cell membranes. Even with stable cavitation alone, the rapidly oscillating surfaces of the bubble create high fluid shear forces that can stress the insonated cells and produce ruptures in the membranes of some types of cells [6].
The acoustic pressure threshold to produce collapse cavitation decreases as the applied frequency decreases, so inertial cavitation is more likely to occur at lower frequencies (given the same intensity or acoustic pressure amplitude). Apfel and Holland observed that the ratio of the acoustic pressure and the square root of the frequency approximately predicted the onset of collapse cavitation for a single acoustic cycle [26]. This has led to the development of a parameter called the “mechanical index” (MI), which is a measure of the likelihood of collapse cavitation occurring. The MI is defined as
where P− is the peak negative pressure amplitude and f is the applied frequency. The onset of collapse cavitation occurs when MI is in the range of 0.3 to 0.4, biological effects start to appear at about 0.7, and detrimental biological effects start to occur when MI > 1 [27]. If multiple acoustic cycles are applied, these threshold values decrease [28].
1.2 Nanoparticles
In this review, a nanoparticle is defined as a distinct collection of molecules that has a size scale between one nanometer and one micron and forms a separate phase in aqueous suspension. Nanoparticles can be classified as solids, liquids or gases, depending upon what phase state the particle possesses as a macroscopic phase at room temperature. Nanosized gas bubbles are used for drug and gene delivery, but their review will not be included in this paper since they are reviewed elsewhere in this same journal issue [3, 24]. In most medical applications, the solid or liquid nanoparticles are dispersed in a liquid, thus forming suspensions or emulsions. For convenience, this review divides these nanoparticles into micelles, nanoemulsions, and suspensions of solid nanoparticles.
1.2.1 Micelles
For the purposes of this review, a micelle is defined as a collection of amphiphilic surfactant molecules that spontaneously aggregate in water into a (usually) spherical vesicle (see Figure 1). The center of the micelle is hydrophobic and therefore can sequester hydrophobic drugs until they are released by some drug delivery mechanism. Conventional micelles are formed from small molecules that have a hydrophilic or polar or charged “head” group and a hydrophobic tail, often composed of the hydrocarbon portion of long fatty acids. The molecular size and other geometrical features of the surfactants determine the size of the micelle [29].
Figure 1.
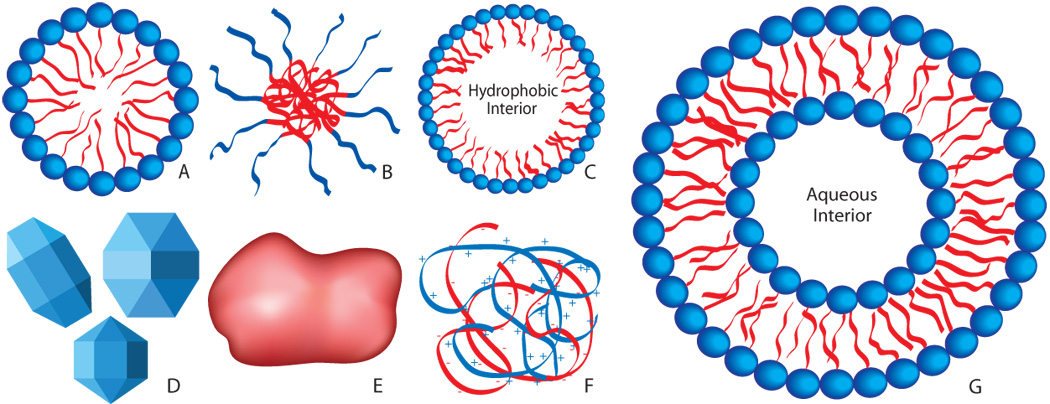
Schematic illustrations (not to scale) of various nanoparticles that may be used in ultrasonic-enhanced drug and gene delivery. A: Micelle (non-polymeric) composed of amphiphilic surfactants. B: Polymeric micelle composed of amphiphilic block copolymers. C: Nanoemulsion consisting of a hydrophobic liquid core stabilized by surfactant. D: Crystalline nanoparticles. E: Amorphous polymeric nanoparticle. F: Condensed ionic oligomers, such as DNA condensed with PEI or cationic lipids. G: Single-walled liposome consisting of an amphiphilic bilayer surrounding an aqueous core.
Polymers that have “blocks” or segments of alternating hydrophilic and hydrophobic character can also form micelles in water. Such polymers used for drug delivery often have hydrophilic blocks composed of poly(ethylene oxide) (PEO) and hydrophobic blocks of poly(propylene oxide) (PPO), poly(lactic acid) (PLA), or other biocompatible and hydrophobic polyethers or polyesters. These copolymers are diblock, triblock, or even more complex structures [30, 31]. One of the most commonly used types of copolymer is an ABA block copolymer of PEO-PPO-PEO structure with trade names of Pluronic® and Poloxamer™.
1.2.2 Nanoemulsions
Emulsions are two-phase mixtures of insoluble liquids, with a “continuous phase” surrounding discrete vesicles of the “dispersed phase”. Usually an emulsion must be stabilized by some kind of surfactant system to prevent the dispersed phase from coalescing into a macroscopic phase [32]. Although many emulsions are of micron size scale, most can also be formulated on the nano-size scale. In drug delivery systems, the continuous phase is most often the aqueous phase, and the drug often is carried in (or is itself) the non-aqueous liquid phase of the emulsion. Surfactant molecules that stabilize nanoemulsions are often the same that form micelles as described in the preceding paragraphs. Hydrocarbon or fluorocarbon liquids are commonly employed as the dispersed phase, which most often carries a hydrophobic drug; thus the nanoemulsion carriers must be dissolved or disrupted somehow for quick release to transfer the drug to the target cells and tissues. More stable emulsions can slowly deliver therapeutics by diffusion from the hydrophobic interior.
1.2.3 Solid Nanoparticles
Solid nanoparticles are distinguished from nanoemulsions by several key features. As defined above, a solid nanoparticle has a solid core, or at least the material in the core forms a macroscopic solid at room temperature. Furthermore, whereas nanoemulsions and micelles normally consist of spherical vesicles, solid nanoparticles are not necessarily spherical. They are often angular, particularly if they consist of crystals of a protein or another therapeutic agent. A surfactant is usually required to stabilize a solid nanoparticle suspension and prevent flocculation.
There are several general categories of solid nanoparticles used in drug delivery [33]. Polymeric nanoparticles consist of non-soluble polymers that are often biodegradable, and thus they can release drugs as they degrade or can release them by diffusion of the drug from the polymeric core. In some formulations the drug or prodrug is attached by a labile linkage to the polymer. If the polymer is not soluble, nanoparticles can be formed. On the other hand, soluble or amphiphilic polymers (with attached drugs) can also be formed into hydrogel nanoparticles by physically or covalently crosslinking the polymer once the nanoparticle has been formed so that it will not dissolve in water (or blood). A rather novel polymeric drug carrier is a dendrimer, a highly branched (usually) hydrophilic single polymer molecule of nanosized scale; drugs can be attached to the ends of the branched polymer arms, or small drugs can be sequestered inside the dendrimer and then be released by diffusion.
There are also many types of non-polymeric nanoparticles. For example, aggregates of hydrophobic proteins or drugs may partially or completely crystallize into nanoparticles. Other examples include nanotubes and fullerenes, which are tubular or spherical cages of graphitic carbon that can carry drugs within their volume or attached to their surface [34].Liposome anoparticles consist of a lipid bilayer surrounding an aqueous core; although often larger than nanoscale particles, they can be produced in sub-micron size, and they can carry drugs in their lipid bilayer or aqueous core. If the lipid is not above its melting temperature, these could be classified as nanoparticles. The review of liposomes for ultrasonic drug and gene delivery is found elsewhere in this journal issue [25].
Solid lipid nanoparticles consist of a solid lipid core that can carry a hydrophobic drug, which is often stabilized by an external monolayer of steric or charged surfactant. Closely related are nucleic acid nanoparticles consisting of DNA or RNA polynucleotides (which carry negative charges) that have condensed with cationic lipids or cationic polymers; once the nucleotide charge is neutralized, the complex is usually sufficiently hydrophobic to form nanosized particles that can be taken up by cellular endocytosis and pinocytosis mechanisms [35]. In this review, free DNA, such as non-complexed plasmids or linear poly(nucleotides), is not reviewed as a nanoparticle because it dissolves in water.
These are just a few of the various nanoparticles that can be used in drug delivery. Although only a small subset of these have been used to date in conjunction with ultrasound, there is no fundamental reason why all of them could not be likewise employed.
2. Micellar Drug Delivery with Ultrasound
The use of micelles in drug delivery is ubiquitous throughout pharmaceutical history. However, only recently has ultrasound been combined with micellar drug delivery. There are many more reports of US-assisted drug delivery from micelles than from nanoemulsions and nanoparticles. Thus we will begin with this topic.
2.1 Micelles in Drug Delivery
Micelles can be formed from simple and small surfactants or from large amphiphilic block copolymers. In applications employing polymeric micelles, hydrophobic drugs are usually sequestered in the micelle core, although it is possible to attach drugs to the hydrophilic polymer of the corona. Most published applications of ultrasonic-assisted micellar drug delivery employ polymeric micelles. There are very few reports of ultrasonically-enhanced gene delivery from micelles.
2.1.1 Polymeric Micelles
The advantages of copolymeric micelles over other types of nanosized drug carriers are:
They are fairly structurally stable at high concentrations of the copolymer (where they form micelles). Since these molecules have high molecular weights, the dissociation time upon dilution is usually longer than other micelles consisting of molecules with lower molecular weight [36].
When their corona contains PEO chains, the micelles are able to circulate in the blood for long times without being recognized and subsequently cleared. They are also stable in other biological fluids [37]. In addition, these polymeric compounds do not degrade, so they have a long shelf-life.
They are usually large enough to escape renal excretion (15–30 nm) while often being of an appropriate size to allow for extravasation and accumulation at a tumor site.
Hydrophobic drugs can be easily incorporated into copolymer micelles by the simple act of mixing. Physical entrapment is an efficient and easy way of loading drugs into micellar systems. Physical entrapment for ultrasonic delivery has been achieved for several anticancer drugs including Doxorubicin (Dox), Ruboxyl (Rb) and Paclitaxel in polymer micelles [38–41].
Another method of incorporating drug into micelles is to use chemical conjugation. Chemical routes involve covalent coupling of the drug to the hydrophobic block of various copolymers leading to micelles of block copolymer-drug conjugates [42–45]. For example, the pharmacokinetics and distribution of Dox in polymeric micelles formed by a drug-polymer conjugate was studied by Kataoka et al. [46]. They found that the conjugate circulated in the form of micelles much longer in blood than when introduced in free drug form. The uptake of drug conjugate by various non-targeted organs proceeded much slower than that of free drug. Other studies by the same group showed that compared to free drug, lower levels of conjugate were found in the heart, lung, and liver, whereas a much higher conjugate level was found in the tumor [47].
Additionally, Pechar et al. have succeeded in synthesizing a biodegradable block copolymer of PEO linked by oligopeptides with amino end groups [48]. They used an interfacial polycondensation reaction to react diamine with poly (ethylene glycol) bis(succinimidyl carbonate). Doxorubicin was attached to the drug carrier by a tetrapeptide spacer of (Gly-Phe-Leu-Gly), which degrades in the body by enzymatic hydrolysis. Polymer-bound Dox was able to delay tumor growth and reduce the toxicity of the drug. However, their approach involved complex synthetic steps as well as lengthy purification procedures and required the chemical (not ultrasonic) release of the drug at the target site.
The main disadvantage with most micellar carriers is their rapid clearance from circulation. However, as mentioned above, by incorporating an exterior layer of PEO, the micelle surface is modified such that they are not cleared as quickly. Because of the hydrophilic nature of PEO, water associates with the PEO chains, and this leads to steric repulsion of proteins and a subsequent reduction in protein adsorption on the surface of these drug vehicles. By reducing, and in some cases preventing, protein adsorption, micellar drug carriers remain longer in the blood circulation because they are protected from detection and subsequent clearance by several biological mechanisms including endocytosis, phagocytosis, and liver uptake [49]. Several research groups reported that the inclusion of PEO chains in the design of drug delivery systems resulted in a dramatic increase in the concentration of encapsulated drug in the blood, which led to a significant increase in drug concentration in tumors [50, 51].Drug delivery using targeting moieties is a popular method being investigated currently to minimize the unwanted side effects of medications. If the blood concentration of the therapeutic agents can be reduced, or even better, if they are kept at a minimum in the systemic circulation while maximizing the drug concentration at the target site, the efficacy of the agent can be maintained while the detrimental side effects observed in the rest of the body can be reduced. Targeting strategies for microbubbles are reviewed elsewhere in this issue [24]. However, many of these same schemes could be applied to micelles used in ultrasonic delivery.
Several research groups have reported on methods of physically (as opposed to covalently) sequestering the therapeutic agent in a molecular vehicle. The contents of these carriers are then released at the target site, thus reducing the negative side effects associated with systemic drug administration of free drug. Ample literature exists in which micelles are used as drug delivery carriers [37, 43–47, 51–67]. Kwon et al. studied the loading of Doxorubicin in micelles of poly(ethylene oxide)-poly(β-benzyl-L-aspartate) [52]. Their work demonstrated that these micelles have solid-like cores that are difficult to load with Dox. Thus, an oil-in-water emulsion is used to sequester the agent in the hydrophobic core of these micelles. The drug is dissolved in chloroform and the resulting solution is allowed to dissolve in the micellar solution. The chloroform is then evaporated leaving the Dox loaded in the micelle at about 5%–12% loading capacity. The loaded nanoparticles are able to release Dox slowly from their cores over a period of several days as measured by UV absorption.
2.1.2 Pluronic Micelles
As mentioned, Pluronics® are a family of ABA type copolymers with A representing the hydrophilic PEO segments, and B corresponding to the slightly hydrophobic PPO mid-segment [53]. Batrakova et al. used the Pluronic copolymers to counteract resistance mechanisms in multidrug-resistant cells [54]. These experiments demonstrated that low concentrations of Pluronic® could overcome multidrug resistance developed by cancer cells that had been repeatedly exposed to a chemotherapeutic agent. In addition to proving that low concentrations of Pluronic® were capable of reversing drug resistance, the group found that higher concentrations of the surface-active triblock copolymer (concentrations above the CMC) resulted in less accumulation of Dox inside the cellular compartments. Thus, they discovered serendipitously that by administering an anti-neoplastic agent in a highly concentrated Pluronic solution, the drug was sequestered inside the hydrophobic core of the resulting micellar solution. The micellar behavior of the Pluronic® copolymer can be employed to sequester hydrophobic drugs, thus reducing the effective drug concentration in the systemic circulation and protecting healthy cells from interacting with the toxic drug. The drug was slowly released from these self-assembled micelles.
It is important to note here that in a high concentration of a Pluronic® solution (above the CMC), equilibrium is established between unimers and micelles. The unimers are still able to overcome the multidrug resistance (MDR) behavior exhibited by the cancerous cells while the presence of micelles is capable of protecting the healthy cells in the body. However, not all block copolymers may be innocuous at such high concentrations.
Micelles in the size range of 5 to 25 nanometers (diameter) are spontaneously formed in solutions of Pluronic® P105, and are very commonly used in ultrasonic drug delivery. In P105 the number of monomers in each PEO and PPO blocks are 37 and 56 respectively. At concentrations above 4%, P105 forms dense aggregates that have the capability of encapsulating hydrophobic drugs [55]. Thus this micellar drug delivery system was very effective is sequestering Dox and then releasing the Dox upon application of low frequency ultrasound [56, 57].
Using KI as a fluorescence quencher, Rapoport et al. [58] have shown that the decrease in DOX and Rb fluorescence, due to its interaction with I−, was considerably less when both drugs where introduced in a solution of 20% P105 than when they were dissolved in PBS, showing that most, if not all, of the drug, was sequestered in the core of the micelles.
2.2 Examples of Ultrasonic Drug Delivery from Micelles
2.2.1 Ultrasound-induced Release from Micelles
To determine if US was releasing drug from the micellar carriers, an ultrasonic exposure chamber with real-time fluorescence detection was constructed to measure acoustically-triggered drug release from Pluronic® P105 micelles under continuous wave (CW) or pulsed ultrasound [56]. Drug release was highest with 20-kHz ultrasound and dropped with increasing ultrasound frequency despite higher power densities. The data suggested that cavitation has an essential role in drug release.
The original hypothesis was that non-thermal non-inertial cavitation caused drug release from unstabilized and stabilized drug carriers (see below), but careful experiments quickly disproved this hypothesis and indicated that inertial cavitation (not stable cavitation) was responsible. Experiments were carried out at 20 kHz or 476 kHz in a custom acoustic chamber equipped with a hydrophone capable of recording the acoustic signature produced by the cavitation events [59]. Results revealed that there is an intensity threshold required to release Dox, and this threshold corresponds to the appearance of acoustic signatures for inertial cavitation: subharmonic emissions and broadband noise [60].
Although Dox is easily sequestered and released from P105 micelles in vitro, this type of micelle is not sufficiently stable to be used in vivo. Dilution in the blood upon injection would quickly dissolve the micelle and prematurely release the drug into the blood stream. Therefore, a second generation drug carrier (NanoDeliv™) that was more stable upon dilution was synthesized from P105 by copolymerizing an interpenetrating network of thermally responsive acrylates in the hydrophobic core [61]. Some third generation micellar drug carriers with time-controlled degradation were synthesized that also release Dox in vitro upon ultrasonication [62, 63].
Using the same fluorescence detection chamber mentioned above, the in vitro release of Dox from several carriers was studied and found that ultrasound causes release of up to 10% of the drug from Pluronic micelles, but only about 3% of the drug in stabilized micelles such as NanoDeliv™ [60]. Although these numbers are low, 3% of drug is released each time ultrasound is applied, so that pulsed US could theoretically release nearly all of the drug when released in the presence of cells that compete for the uptake of Dox.
One of the puzzling aspects of the research was that even though inertial cavitation was observed at 476 kHz, there was no observation of change in fluorescence indicative of Dox release from Pluronic micelles at this higher frequency, even while using the same laser detection system employed so successfully at 70 and 20 kHz. Thus, to determine why and how bubble cavitation activity at this frequency was different than at 70 kHz, bubble oscillation behavior was modeled using the Keller-Miksis-Parlitz equation [64]. The results showed that at 476 kHz, the bubble takes a classical “period-doubling” route to chaotic oscillation over the range of mechanical index (MI) = 0.28 to 0.41, whereas a bubble at 70 kHz jumps quickly to chaotic behavior at MI =0.35 [65].
To further understand ultrasonically induced Dox release from micelles, mechanistic models were fit to the kinetic data of Dox release and re-encapsulation from stabilized and unstabilized micelles subjected to long pulses of ultrasound. The simple models using a limited data set showed that Dox release was zero-order with respect to the encapsulated drug concentration, and that re-encapsulation was first-order with respect to free (released) Dox concentration [66]. Subsequently more data was collected to further expand the ranges of operating conditions including power density, frequency, Pluronic P105 concentration, and temperature. The data on Dox release were analyzed as a function of these various acoustic parameters, applying methods of both artificial neural network models [67] and chemical kinetic models [68]. The results of these models showed that drug release is most efficient at lower frequencies. The analysis also demonstrated that the amount of drug release increases as the power density increases. Sensitivity plots of ultrasound intensity revealed a drug release threshold of 0.015 W/cm2 (MI = 0.15) and 0.38 W/cm2 (MI = 0.40) at 20 and 70 kHz, respectively [60]. The presence of a power density threshold provides further evidence that inertial cavitation plays an important role in acoustically-activated drug release from polymeric micelles.
Further studies carefully examined the onset of collapse cavitation at 70 kHz and its effect on drug release from micelles. In these experiments, the release of Dox in the diagnostic volume was measured by a laser fluorescence technique described previously [59]. Simultaneously the acoustic spectrum of the bubbles within the same diagnostic volume was recorded using a calibrated hydrophone (Bruel and Kjaer 8103, Decatur, GA) whose output voltage was monitored and captured with an oscilloscope. The signal was analyzed online with a spectrum analyzer or post-processed by Fourier transformation to produce an acoustic spectrum. The observation of harmonic frequencies (2f, 3f, etc.) of the applied frequency (f) revealed the presence of gas bubbles that were excited into large amplitude non-linear oscillation [69]. Subharmonics also appeared at higher acoustic intensities, indicative of highly non-linear oscillations thought to indicate the onset of inertial cavitation. Most importantly, the sudden appearance of a broadband noise background was attributed to white noise from shock waves produced by the onset of inertial cavitation. The results of these experiments showed that there is a strong correlation between the subharmonic signal and the amount of Dox released, suggesting that chaotic bubble behavior and inertial cavitation are highly associated with the observed drug release [60].
2.2.2 In vitro Drug Delivery Targeting Cells
An early study of ultrasonic-enhanced micellar drug delivery involved DNA damage induced by Doxorubicin delivered to Human leukemia (HL-60) cells from Pluronic® P105 micelles with and without the application of ultrasound. This research employed the comet assay [70], which revealed the amount of DNA damage due to Dox delivery. Cell viability was also measured, and is shown in Figure 2. These results showed remarkable and beneficial synergism between US, Dox and Pluronic® micelles. In the absence of US, the cells were protected from Dox by the Pluronic®; but when US was applied, the rate of cell killing was greater than that produced by Dox alone. The US was acting as a switch. The comet assay revealed that the mode of cell death in this ultrasonic drug delivery system was apoptosis rather than necrosis [71]. The fact that apoptosis is implicated suggests that the released Dox had induced gradual DNA fragmentation as opposed to the alternative hypothesis that US caused severe membrane damage and subsequent cell death by necrosis. In other words, US did not kill the cells by membrane damage, but it enhanced the typical action of the Dox.
Figure 2.
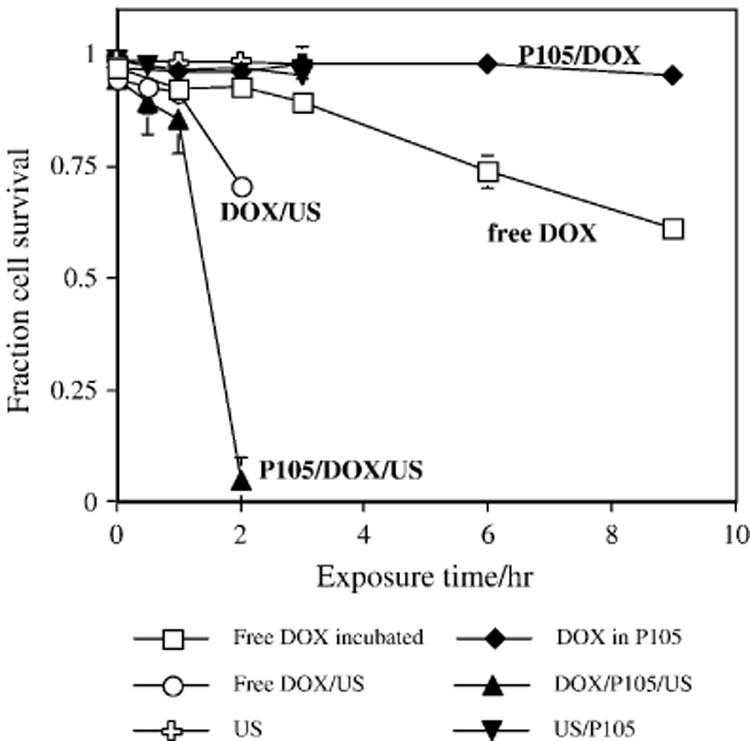
HL-60 cell viability determined by Trypan Blue exclusion after exposure to the following conditions □: 10 µg/ml DOX in PBS; ◆: 10 µg/ml DOX in 10% Pluronic;○: 10 µg/ml DOX in PBS with ultrasound; σ: 10 µg/ml DOX in 10% Pluronic with ultrasound; 9: PBS with ultrasound only; τ: 10% Pluronic with ultrasound. Error bars represent standard deviations. Where error bars are not observed, the size of the error bars is smaller than the size of the symbol. Figure used by permission from reference [57].
To verify the role of cavitation bubbles in micellar delivery, Stringham et al used an in vitro cell model to study the effect of pressurizing the acoustic chamber to suppress the effects of cavitation bubbles. In general, as the ambient pressure is increased at constant US intensity, there is a decrease of cavitational activity, which in our system manifests itself by a reduction in the height of subharmonic and ultraharmonic peaks in the acoustic spectrum [72]. Such suppression would lead to a reduction in the shear stress in the environment of the micelles. In this study, the reduction in shear stress with static pressurization was inversely correlated with the uptake of a model drug, calcein, by rat colon cancer cells in vitro [73]. Calcein, which normally does not penetrate healthy cell membranes, accumulated inside the cells during ultrasonic exposure at 476 kHz. However, as the chamber was pressurized to 20 reduce inertial cavitational activity, the amount of calcein uptake correspondingly decreased, thus directly linking inertial cavitation to drug uptake by cells.
2.2.2 In vivo Drug Delivery Targeting Tumors
Our research group has been using US to enhance the delivery of Dox from stabilized micelles to solid tumors in a rat model [74, 75]. Using a bilateral model of a subcutaneous tumor on each hind leg, one tumor was exposed to 20 or 70 kHz US, but both tumors received the same systemic Dox exposure, either free or encapsulated in NanoDeliv™. Results showed that tumors in the US-treated leg grew slower or regressed, compared to the untreated leg (p < 0.0061). In a more recent study using the same model but with 500 kHz US, we showed similar tumor regression in the US-treated leg (p < 0.001) even though there was only a slightly greater amount of Dox in that tumor tissue [76]. Because the slight increase in Dox concentration correlated with a large decrease in tumor growth, we postulated that more is happening than simple Dox release, and that perhaps cell membranes were being stressed in vivo similarly to the in vitro situation.
Howard et al. reported the use of a micellar paclitaxel delivery system with US to treat a drug resistant breast cancer tumor cell line [41]. Their results showed a 20-fold increase in drug uptake when comparing sonicated and non-sonicated samples. Furthermore, the injection of this paclitaxel formulation in conjunction with acoustic activation resulted in the complete regression of the MCF7/ADm tumors in nu-nu mice. Mice were injected with the drug formulation for 3 consecutive weeks, with the tumors receiving 30 seconds of 1 MHz ultrasound at a power density of 3.4 W/cm2. Recently, Rapoport summarized the current physical methods being investigated to deliver chemotherapeutic agents from physical micelles to cancerous tissue [77].
Myhr et al applied ultrasound to mice inoculated with a human colon cancer cell line to deliver fluorouracil encapsulated in a stabilized micelles similar to NanoDeliv™ [78].Their results showed a significant reduction in the tumor volume compared to the group not receiving ultrasound. The authors reported a more significant tumor volume reduction at higher drug concentrations.
2.3 Mechanisms of Ultrasonic-enhanced Uptake from Micelles
Drug release from the nanoparticles described above is caused by the cavitational oscillation and collapse of gas bubbles in the ultrasonic field. We have studied the physics of the interaction between the oscillating gas bubbles and the nanoparticles and have established thresholds of acoustic intensity that produce sufficient shear on the particles to produce drug release [59, 60, 67]. We presume that there is also significant shear on cell membranes that contribute to some of the observed biological effects.
The question remains as to how ultrasound enhances uptake of DOX by the cell, both in vitro and in vivo. In this section, we will discuss three postulated mechanisms that have been tested in an attempt to illuminate the process of ultrasonic-enhanced drug delivery from the micelle to the cell. These postulates are 1) ultrasonic release of the drug from micelles is followed by normal transport into the cell; 2) ultrasound upregulates endocytosis of the micelles (with their drug cargo) into the cell; 3) ultrasound perturbs the cell membrane which increases passive but direct transport of the drug, either released or still encapsulated, into the cell cytosol.
The first hypothesis proposes that the drug is released from micelles outside the targeted cells, followed by normal transport of the drug into the cells by simple diffusion or normal cellular uptake mechanisms such as endocytosis. Simply put, there is a greater concentration of drug outside the cell, so more drug gets inside the cell by normal processes, whatever they may be. To test this postulated mechanism, some of the hydroxyl groups present at the ends of Pluronic® P105 forming the micelles were labeled with a fluorescein derivative that fluoresces at a different wavelength (550 nm) than does Dox (590 nm) [79–81]. HL-60 cells were then incubated or sonicated with Dox sequestered within the fluorescein-labeled P105 micelles. The results showed that both DOX and the labeled P105 appeared inside the insonated cells.
The next question was whether the fluorescently-labeled P105 entered the cells along with or independent of the DOX. Fluorescent measurements revealed the presence of P105 inside the cells both when incubated (no insonation) and when insonated for 20 minutes. Unfortunately the fluorescent measurements were not sufficiently quantitative to indicate whether insonation changed the ratio of Dox to P105 that eventually was found in the cells. Because the labeled P105 molecules themselves were found inside the HL-60 cells, there was rejection of the first hypothesis that US only caused external drug release from the micelles followed by passive drug diffusion (drugs only) through the cell membrane without the accompanying diffusion of P105 into the cells. Although these experiments showed that the P105 entered the cells, it was not possible to determine whether the copolymer (with or without drug) entered the cells through holes created in the cell membrane or through endocytotic routes.
The second hypothesis was that ultrasonic exposure upregulates endo-/pino-cytosis such that entire micelles with drug are endocytosed into the cells. Since it is unlikely that HL-60 cells express receptors for Pluronic micelles or their exterior PEO chains, receptor-mediated endocytosis was considered to be very unlikely. However, micelles might be transported into the cells by pinocytosis, a non-specific form of endocytosis in which small volumes of external liquid are engulfed by membrane invaginations. The pinocytotic vesicles eventually fuse with lysosomes. To test the postulate that insonation upregulated pinocytosis and therefore increased the uptake of the Dox and P105, we employed a model drug, namely Lysosensor Green, which fluoresces more strongly in an acidic compartment such as a lysosome [80]. The great majority of endosomes fuse with primary lysosomes to form secondary lysosomes, which have a pH of about 4.8, compared to the cytosol pH of about 7.1. Cells exposed to US and P105 micelles with Lysosensor Green were examined by flow cytometry, and they showed no statistically significant difference in fluorescence between cells incubated (no US) and cells insonated for 1 hour at 70 kHz. Thus, ultrasound did not cause the probe to partition to a more acidic environment any more than it did without ultrasound, and the hypothesis was rejected that US induces upregulation of endo-/pino-cytosis in this cell line.
Rapoport et al. reported that micellar drug delivery with ultrasound enhanced the rate of endocytosis into ovarian carcinoma cells [82–84]. Furthermore, Sheikov et al. showed similar results, namely that US induced pinocytosis in the endothelial cells [85]. Thus the role of US in promoting endocytosis and pinocytosis is unresolved. It is possible that different cells respond differently to the ultrasonic stimulus such that a general rule cannot be applied.
The third hypothesis, that drug-laden micelles enter through holes in the membranes of insonated cells, is supported by several observations. For example, the study testing the first hypothesis above showed that both Dox and Pluronic enter the cell and that Dox entry is enhanced by US; however, that enhanced entry cannot be attributed to upregulated endo-/pino-cytosis. Furthermore, studies with the model drug calcein (see section 2.2.2 above) indicated that insonation compromises the cell membrane sufficiently that hydrophilic molecules can enter – molecules like calcein that are normally excluded. This entry is directly related to bubble cavitation activity. Finally, there are other reports, including those with electron micrographs of damaged cells, that support the hypothesis that US creates transient holes in the cell membrane [7, 15, 17, 18, 86]. The ability of carefully controlled US to create non-lethal and repairable holes in cell membranes is gaining support. For example, Schlicher et al. have shown that the accumulation of the model drug calcein in prostate cancer cells was caused by US-induced membrane disruptions [7]. In many cases these disruptions can be repaired and are non-lethal.
Prentice et al has shown examples of single cavitation events that created large divots in cell membranes [86]. They used a burst of 1 MHz ultrasound to cause the collapse cavitation of a single microbubble adjacent to a cell layer. Subsequent atomic force microscopy of the surface revealed a crater or hole of 16 µm in diameter and ~1 µm in depth.
Tachibana et al. has reported the increase in cell membrane permeability and skin porosity caused by ultrasound [15, 17, 18, 87–92]. His group has shown that the exposure of HL-60 cells to 255 kHz of ultrasound and MC 540 (an anticancer drug) for 30 seconds formed pores in the cell membrane [15]. The cytoplasm of some cells seemed to have extruded through the pores formed in the cell membrane as a result of sonoporation. When cells were exposed to ultrasound alone, the cell membrane showed some minor disruptions. Saito et al. [16] demonstrated that exposure to ultrasound increased the permeability of corneal endothelium cells. The increase in permeability appeared to be reversible and the cells regained their membrane integrity after several minutes.
Thus there is ample evidence that ultrasonic cavitation events create transient holes in the cell membrane, which in the case of micellar drug delivery would increase the passive diffusion of micelles and drugs into the cells.
Having rejected postulated mechanisms 1 and 2 as the sole or primary mechanism for ultrasonic-enhanced drug uptake from micelles, there is ample evidence that the third proposed mechanism is the major player, the mechanism of ultrasound-induced permeation of cell membranes followed by direct transmembrane transport of micelles and extracellular Dox. It is still possible that mechanism 1 (drug release from micelles and subsequent normal transport) and mechanism 2 (upregulated endocytosis) may play a small but less significant (and less measurable) role. This scenario is consistent with the in vivo observations that insonation of a tumor with Dox-loaded micelles produced only a slight increase in the Dox concentration in rat tumors; and this occurred only at the shortest (30-minute) time points. Yet the tumor growth was significantly retarded by this therapy [76]. Furthermore, these results were obtained using stabilized micelles that only release about 3% of their Dox cargo when insonated in vitro [60]. Thus, although ultrasound does appear to release drug from micelles, such release may not be the most important factor in reducing tumor growth in vivo. Cell membrane perturbation may be of equal or greater importance.
3. Ultrasonic Gene Delivery from Nanoparticles
Although most reports of US-assisted DNA and RNA delivery involve the presence of microbubbles, there are some publications which report condensation of the nucleic acids with cationic lipids and polymers, followed by application of US in the absence of bubbles to effect gene transfection. It is noteworthy that in the absence of microbubbles or other acoustically active agents, ultrasound still increases gene transfection of naked or condensed DNA [89–91, 93–95]. There is a plethora of literature on transfection with cationic materials in the absence of ultrasound that cannot be reviewed here. The reader is referred to some recent reviews [35, 96–98].
Compared to free plasmid delivery, there are at least three advantages to condensing polynucleotides with cationic lipids and polymers in ultrasonic gene delivery. First, the plasmid appears to be protected from degradation by ultrasound when complexed. For example, Kuo et al found that condensation by addition of polyethylene glycols offered little protection from degradation by 20 kHz ultrasound, while condensation with sufficient amounts of high MW polyethyleimine and poly-L-lysine protected the plasmids from degradation [99]. Zobel et al found that plasmids adsorbed on nanoparticles of poly(monomethylaminoethylmethacrylate) were severely degraded by 35 kHz ultrasound; however, plasmids adsorbed on diethylaminoethyldextran-stabilized polyhexylcyanoacrylate nanoparticles were only slightly degraded under the same conditions [100]. Apparently the character of the substrate makes a difference, and interaction between the charge groups appears to be essential in providing protection. Unfortunately some of the cationic polymers used in gene condensation for transfection can be toxic at high concentrations [101, 102].
A second advantage of complexation is that it protects the plasmid from enzymatic degradation in the blood plasma, thus allowing more time for extravasation [103–105].
Thirdly, when plasmids are condensed with cationic lipids or cationic polymers, the resulting nanoparticles usually range in size from 100 to 400 nm [106–110]. Similar to micelles and nanoliposomes, these nanoparticles are expected to extravasate into tumor tissue, to enter cells through small membrane ruptures, and to be endocytosed. The smaller sized nanoparticles may also escape clearance by the reticuloendothelial system (RES).
3.1 Nanoemulsions for gene delivery
Interestingly, there are few reports of ultrasonically-assisted gene transfection that employ liquid nanoparticles other than nanoliposomes, which are readily used because the nucleic acids are soluble in their aqueous interior [25]. Furthermore, most of these reports have employed perfluorocarbon liquids instead of hydrocarbon liquids.
Unger et al reported the use of low-boiling-point perfluorocarbons such as perfluorohexane and perfluoropentane to transfect cancer cells in vitro using ultrasound [111, 112]. They proposed that plasmids condensed into a solid phase that was suspended inside the perfluorocarbon interior phase of their nanoemulsions, and they provided some transmission electron microscopy to support their claim [113]. In subsequent insonation, they claimed that perfluorohexane was transformed to a gas bubble that subsequently undergoes cavitational activity to enhance the transport of the plasmid into the cells by less understood mechanisms. Unger also taught that perfluoropentane, which vaporizes at 29°C (below body temperature), could be similarly employed as a liquid nanoemulsion. The additional LaPlace pressure imposed by the high curvature of the tiny nanoparticle would keep the liquid from boiling at body temperature. Although first presented in the late 1990’s [114], this nanoemulsion system for gene delivery has not been developed much since that time. It has potential for use, but perhaps the difficulty in formulation and nanoemulsion stability has discouraged further study.
In a rather unique study employing the abovementioned perfluorocarbon nanoemulsions, Lee et al employed 1 MHz US to transfect a luciferase gene into a silkworm model [115]. The creative aspect of this study was the combination of various modalities: transfection employing 1) a cationic liposome without US, 2) a liquid fluorocarbon nanoemulsions with US and 3) a combination of the liposome and nanoemulsion with US. These formulations were injected into the haemocoel of the silkworm larva. Their results showed that optimal transfection occurred at 60 seconds of insonation, and the formulation that contained both liposomes and nanoemulsion produced the most transfection. They hypothesized that the ultrasound transformed the perfluorohexane in the nanoemulsions into gas bubbles that caused “sonoporation” of the cells in the larva, thus allowing direct gene transfection through transient membrane “holes”.
Rapoport et al were able to use multifunctional nanoparticles in conjunction with perfluoropentane microbubbles to deliver Doxorubicin to MDA MB231 breast tumors both in vitro and in vivo [77]. This study reported a statistically significant tumor regression when nanoparticles and nanoemulsions were used with ultrasound. They argued that in addition to acting as drug delivery vehicles, these nanovehicles could be used in diagnosing cancerous tissue.
3.2 Nanoparticles for gene delivery
3.2.1 Lipid-based nanoparticles
Gene transfection by complexes of cationic lipids and DNA plasmids, sometimes called lipofection, has been studied for years. There are commercially available systems that are optimized for transfection of various cell lines. However, there has been relatively little combination of ultrasound with lipofection, possibly because early studies using ultrasound and gas bubbles showed that the addition of the contrast agents enhanced transfection of naked DNA much more than traditional transfection using the more time-consuming lipofection process, which occurs through endocytosis and pinocytosis mechanisms [93]. The incubation time to produce gene expression is also slower using only lipofection than when using naked DNA and contrast agents [116, 117]. So why bother with lipofection when contrast agents are available? In the few studies that combined US and lipofection, the results were not always positive. Koch et al studied the transfection of brain tumor cells using 2 MHz pulsed US and LipofectAmine™ (Life Technologies) condensed with plasmids coding for green fluorescent protein (GFP). Application of 1 minute of US in the absence of contrast agent produced no change in transfection efficiency compared to conventional lipofection alone [118].
To summarize this section on ultrasonic-enhanced lipofection, it appears that lipofection is not enhanced by ultrasound unless gas bubbles are present. If gas bubbles are present, then the transfection by naked DNA appears to be sufficient in vitro. However, one must remember that there are advantages with respect to enhanced durability when plasmids are complexed with cationic lipids.
3.2.2 Polymeric nanoparticles
Solid polymeric nanoparticles in an ultrasonic field, even in the absence of preformed gas bubbles, appear to be effective in nucleating bubble formation that leads to a reduction in the cavitation threshold in water [119–121]. For example, polystyrene (PS) nanoparticles decreased the threshold of ultrasound-induced cavitation activity in pure water from about 7.3 bar to less than 5 bar, depending upon the size and concentration [122–124]. The threshold decreased with increasing particle concentration, and decreased with decreasing particle size [124]. Thus even without the use of gas bubble contrast agents, there was sufficient cavitational activity to produce significant bioeffects. Although other investigators have used polymer and polyplex nanoparticles (other than PS), they did not report whether these particles lowered thresholds or enhanced US activity. It would be very beneficial to know if this phenomenon extends to other types of solid nanoparticles, the mechanisms by which this occurs, and whether such “threshold lowering” also occurs in blood or in intracellular liquids. Polymers commonly used for drug and gene delivery include PS, poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), and polyplexes of plasmids and cationic polymers [122–132].
The advantage of polymeric nanoparticles over gas bubbles as enhancers of cavitational activity is that nanoparticles can extravasate beyond the capillaries where the gas bubbles cannot go because of their size. Once there, nanoparticles might catalyze cavitational activity. For example, tumor-bearing mice were injected with a PS suspension which was allowed to extravasate for one day; then 20 kHz US was applied to one tumor but not the other [125]. Histological results showed that the tumor vasculature was disrupted in the insonated tumor, but there was no damage to the non-insonated tumors (with PS particles) or tumors that were insonated without PS nanoparticles. In a similar study monitoring tumor growth rate, the tumors with PS nanoparticles regressed in volume for about five days following 20 kHz insonation, but then continued to grow at the normal rate thereafter [122].
3.2.3 Examples of gene delivery
The group of Hosseinkhani et al has published several papers showing very effective ultrasonic-enhanced gene delivery using polyplexes of DNA and cationic-derivatized natural polymers, such as cationized dextran [131] and gelatin [128–130, 132]. In these experiments, 3 MHz US (2 W/cm2, 10% duty cycle) was applied for 1 to 2 minutes transdermally to various tumors or to muscles on mice. Insonation always enhanced gene expression for a few days. The authors speculated that cavitation-induced cell membrane damage and permeation were responsible for the enhanced genetic expression.
In a recent study, Chumakova et al created polyplexes of reporter gene DNA and polyethyleneimine (PEI) and loaded them on 300-nm-diameter PLGA nanoparticles [127]. These particles were injected into nude mice bearing bilateral human prostate tumors on their back. Pulsed insonation for 5 minutes at 1 MHz and -7 bars produced a significantly greater expression of the reporter gene in the tumor compared to the non-insonated bilateral control tumor. The authors believed that the particles were “gas-filled” and oscillated in the acoustic field, which stimulated their uptake by endocytosis. This reviewer believes that it is also possible that the particles induced gas bubble formation and subsequent cavitational activity that could also have promoted uptake.
In an interesting combination of nanoparticles and gas bubbles, Zhou et al. [126] created reporter gene polyplexes with poly(L-lysine) (PLL) and with reducible PLL (rPLL); then they further stabilized these against RES clearance with a coating of poly(N-(2-hydroxypropyl) methacrylamide). These nanoparticles were mixed with a suspension of 4T1 carcinoma cells and Optison contrast agent in vitro and exposed to 2.25 MHz CW ultrasound (0.6 MPa) for 1 minute. Insonation increased the gene expression from the nanoparticles by 2 orders of magnitude, but increased by 3 orders of magnitude the gene expression from naked DNA. Although delivery of naked DNA outperformed the nanoparticles, the authors point out that naked DNA would be cleared very rapidly in vivo, and thus the nanoparticles would have the advantage of persistence and extravasation.
3.3 Ultrasonic-assisted Gene Delivery with Micelles
Chen et al. reported the use of Pluronic® copolymers in gene delivery [133]. Their study included three different compounds, namely L61, F127 and P85, and employed 1 MHz US for 20 s at a power density 1 W/cm2. They concluded that both F127 and P85, at concentrations ranging between 0.005% and 0.1%, were efficient in increasing the rate of transfection up to 30% when US was employed. Since polynucleotides are not hydrophobic, this reviewer questions whether the plasmids were within the micelle core, were associated with the PEO corona, or were just free plasmids taking advantage of a lower cavitation threshold induced by the Pluronic® micelles.
4. Future
4.1 Questions regarding mechanisms
Despite the abundance of research on ultrasonic activated drug and gene delivery, there yet remain plenty of unanswered questions and potential improvements that should keep researchers busy for several more years.
Perhaps the foremost question that researchers should address is whether ultrasonic delivery from micelles has any advantage over delivery using microbubbles such as contrast agents. Cavitation is definitely involved in micellar drug delivery that does not use contrast agents, but what is the source of the bubbles? Are these mysterious bubbles nucleated and grown to a uniform and predictable size and density, or are they random and haphazard, thus potentially creating random and haphazard results? Since the size distribution is not known, it is difficult to predict the ultrasonic frequency that would be most efficient to produce the desired type of cavitation. The addition of stabilized microbubbles (such as contrast agents) has the definite advantage of presenting a bubble population with a known size and concentration, so they can be modeled mathematically or at least correlated with various results. Micelles, however, can carry and release hydrophobic drugs, which bubbles can do only if there is an oily layer also present in the bubble, as has been demonstrated by Unger’s lipospheres [134]. Micelles also have a strong advantage over bubbles because of their size. They are not cleared as quickly and they can extravasate into some tumors.
Another disadvantage of micelles is that they have no convenient method of sequestering and delivering DNA and RNA. Thus their potential use in gene delivery is small or perhaps non-existent. One might as well use the more controlled and proven microbubbles, although there is still a problem with the lack of extravasation.
Nanoparticles composed from positively-charged lipids and polymers could have a tremendous advantage over micelles in the area of ultrasonic gene delivery in situations where bubbles could not be used. As mentioned, microbubbles cannot extravasate beyond the endothelium in the circulatory system, except perhaps in tumors with extremely large gaps in the endothelium.
The observation by Larina et al [122] that solid nanoparticles reduce the cavitation threshold in water is fascinating and generates the question of whether such a threshold reduction would also occur in intracellular fluid. The obvious, easy, and fairly useless answer is “probably so”, but the real issue is “how much”. One can also ask the same question for micelles: “Do micelles also reduce the cavitation threshold?” The general theory of colloids and gas bubble nucleation confidently state that the answer is yes, but again, hard numbers on the extent of threshold reduction are missing. Do all micelles lower the threshold equally, or do the chemical details of the surfactant molecules play an important role? On the other hand, perhaps this topic has not been vigorously studied because it may not be important in the larger scheme of drug delivery. One should question whether a lower threshold is necessary to improve targeted drug delivery. Usually lower acoustic energies are correlated with less cell damage, but less damage is also attributed to less energetic cavitational activity. So lowering the threshold with particles to produce less cell damage may also produce less gene and drug delivery.
A related question is whether these polymeric and surfactant micelles naturally scavenge and sequester gas molecules dissolved in the liquid phase. If so, are investigators inadvertently using gas filled micelles, which are a form of nanobubbles, and would they follow classical bubble behavior with their very high surfactant to gas ratio and very small radius of curvature? This is another area ripe for study, both theoretically and experimentally.
So much for the esoterical questions that may or may not have any bearing on clinical applications of ultrasound in drug and gene delivery. The questions that the clinicians pose are much more practical, such as what drugs can be carried by nanoparticles, and what are the loading levels. Other practical questions include how to attach active targeting moieties to the nanoparticles and whether active targeting is really needed if one can just focus ultrasound on the target site. Perhaps most important are questions related to the toxicity of the nanoparticles and/or their degradation products. It seems as if there are more questions than answers, and hopefully these questions will stimulate creative research to improve ultrasonic drug and gene delivery.
4.2 Potential for Therapeutic Use
With all of the unanswered questions and possible pitfalls mentioned above, one may ask what is the potential for therapeutic use of such system within the next few years. Without a crystal ball we can only guess; but our guess would be that there will be a great competition in the area of ultrasonic gene delivery between microbubbles, which are already well accepted in the clinic, nanoliposomes, and nanovehicles, particularly nanoemulsions and solid nanoparticles, which are newcomers. We predict that while nanobubbles may win the competition in the realm of gene delivery to cells within a layer or two of the circulatory system, nanovehicles will find their niche in gene delivery to tumors in which they can extravasate.
Micelles, with their limited stability and hydrophobic interior, may never find a place in gene delivery. However, they have great potential for ultrasonic-activated delivery of hydrophobic drugs. We foresee possible uses beyond just cancer chemotherapy. Other potential applications include localized delivery of pain medications, either to the source of the pain signals, or perhaps to the nerves carrying the pain signals. Another potential application is in the localized delivery of steroids for anti-inflammatory therapy, or in the delivery of hormones or signaling peptides.
One of the limitations of ultrasonic delivery from nanovehicles has been that much of the early research was conducted at very low frequency (<250 kHz), and such low frequency is difficult to focus into small volumes. However, we think that the field of high intensity focused ultrasound (HIFU) will continue to develop and produce transducers and supporting equipment that can be used in conjunction with these nanovehicles.
To summarize the future of ultrasonic drug and gene delivery with the various types of nanoparticles, there is already a foundation in place, but the edifice has yet to be built. Each type of nanovehicle will find its particular niche in therapeutic medicine, and there will be strong competition between microbubbles, nanoemulsions, and nanoparticles for gene delivery. Obviously there is still much research to be done.
5. Summary
Several recent review articles have summarized the new advances in polymer genomics [53, 135], nanoparticles used in drug delivery [136], polymers used in cancer therapy [137] and therapeutic ultrasound [20, 22, 23, 138]. Here we have reviewed the recent advances in the use of micelles, nanoparticles, liquid emulsions, liposomes, and shelled vesicles in combination with ultrasound in drug delivery. Based on the above-mentioned advantages of both acoustic power and nanocarriers, it is probable that both research areas will experience considerable expansion in quantity and scope.
Nanoparticles have played and will play a major role in ultrasonic drug and gene delivery, particularly in delivering the therapeutic to the targeted delivery site. The major advantage that nanoparticles have over microbubbles is that they can be made small enough to extravasate effectively from the leaky vasculature of some tumors. Their advantage over nano-liposomes is that they can sequester and deliver hydrophobic drugs. Some disadvantages include their often-challenging preparation and instability. Toxicity should always be addressed, as is the case with any drug delivery vehicle.
Currently, the most commonly used nanoparticles with ultrasound are polymeric micelles. Although not yet in the clinic, there is more published on ultrasonic delivery with polymeric micelles than other nanoparticles reviewed herein (excluding nanoliposomes and gas bubbles). Most of the polymers employed in these micelles are polyether block copolymers, particularly those with hydrophilic blocks of polyethylene oxide. Ultrasound releases hydrophobic drug from these micelles, most probably by shear effects from bubble cavitation. Ultrasound appears to create transport pathways through the cell membrane by which drugs and genes can enter the cytosol. The role of US in upregulating endocytosis is still under investigation.
Micellar drug delivery has been effective in rat and mouse cancer models employing ultrasound between 20 kHz and 1 MHz of relatively low intensity. Micelles could probably find even more use when combined with additional strategies such as conjugation with targeting ligands and concurrent deployment with microbubbles. Ultrasonic gene delivery employing micelles is still in its infancy (and may remain there).
Nanoemulsions have been used to a small extent for both drug and gene delivery with ultrasound, particularly emulsions of perfluorocarbon liquids such as perfluoropentane and perfluorohexane. These liquids have very high vapor pressures and are thought to transform to gas bubbles during the low-pressure phase of the acoustic cycle, thus proffering the advantages of bubbles in a smaller nano-sized package.
Like with nanoemulsions, ultrasonic gene delivery with solid nanoparticles is also in its infancy. Some solid polymeric nanoparticles appear to reduce the cavitation threshold, but the mechanism behind this observation is unproven. Cationic polymers are used to condense DNA, and their efficiency in gene delivery is greatly enhanced by the presence of microbubbles. Drugs have been incorporated into degradable polyester nanoparticles that have been used in conjunction with US.
There remains a great deal of research to be done and important questions to answer on the pathway to clinical application of ultrasonic drug and gene delivery from nanoparticles, the most important of which is the question concerning the acoustic parameters to be used. The research and theory that applies to microbubble interactions with ultrasound may not apply to nanoparticles. Despite the long research road ahead, their prospect is bright for beneficial applications in medicine.
Acknowledgments
Authors would like to acknowledge funding from the National Institutes of Health (CA-98138).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brennen CE. Cavitation and bubble dynamics. New York: Oxford University Press; 1995. p. 282. [Google Scholar]
- 2.Wu JR, Nyborg WL. Ultrasound, cavitation bubbles and their interaction with cells. Advanced Drug Delivery Reviews. 2008 doi: 10.1016/j.addr.2008.03.009. accepted. [DOI] [PubMed] [Google Scholar]
- 3.Sboros V. Response of contrast agents to ultrasound. Advanced Drug Delivery Reviews. 2008 doi: 10.1016/j.addr.2008.03.011. accepted. [DOI] [PubMed] [Google Scholar]
- 4.Leighton TG. What is ultrasound? Progress in Biophysics & Molecular Biology. 2007;93:3–83. doi: 10.1016/j.pbiomolbio.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Nyborg WL. Biological effects of ultrasound: Development of safety guidelines. Part ii: General review Ultrasound Med. Biol. 2001:301–333. doi: 10.1016/s0301-5629(00)00333-1. [DOI] [PubMed] [Google Scholar]
- 6.Rooney JA. Hemolysis near an ultrasonically pulsating gas bubble. Science. 1970;169:869–871. doi: 10.1126/science.169.3948.869. [DOI] [PubMed] [Google Scholar]
- 7.Schlicher RK, Radhakrishna H, Tolentino TP, Apkarian RP, Zarnitsyn V, Prausnitz MR. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound in Medicine and Biology. 2006;32:915–924. doi: 10.1016/j.ultrasmedbio.2006.02.1416. [DOI] [PubMed] [Google Scholar]
- 8.Goss SA, Johnston RL, Dunn F. Comprehensive compilation of empirical ultrasonic properties of mammalian tissues. Ii. J. Acoust. Soc. Am. 1980;68:93–108. doi: 10.1121/1.384509. [DOI] [PubMed] [Google Scholar]
- 9.Goss SA, Johnston RL, Dunn F. Comprehensive compilation of empirical ultrasonic properties of mammalian tissues. J. Acoust. Soc. Am. 1978;64:423–453. doi: 10.1121/1.382016. [DOI] [PubMed] [Google Scholar]
- 10.Loverock P, Ter Haar G, Ormerod MG, Imrie PR. The effect of ultrasound on the cytoxicity of adriamycin. Brit. J. Radiol. 1990;63:542–546. doi: 10.1259/0007-1285-63-751-542. [DOI] [PubMed] [Google Scholar]
- 11.Saad AH, Hahn GM. Ultrasound enhanced drug toxicity on chinese hamster ovary cells in vitro. Cancer Res. 1989;49:5931–5934. [PubMed] [Google Scholar]
- 12.Saad AH, Hahn GM. Ultrasound-enhanced effects of adriamycin against murine tumors. Ultrasound Med. Biol. 1992;18:715–723. doi: 10.1016/0301-5629(92)90122-q. [DOI] [PubMed] [Google Scholar]
- 13.Saad AH, Hahn GM. Ultrasound enhances adriamycin toxicity in vitro. In: Chato JC, Diller TE, Diller KR, Roemer RB, editors. Heat transfer in bioengineering and medicine. New York: Am. Soc. Mech. Engr. Press; 1987. pp. 28–31. [Google Scholar]
- 14.Saad AH, Williams AR. Effects of therapeutic ultrasound on clearance rate of blood borne colloidal partricles in vivo. Br. J. Cancer. 1982;45:202–205. [PMC free article] [PubMed] [Google Scholar]
- 15.Tachibana K, Uchida T, Tamura K, Eguchi H, Yamashita N, Ogawa K. Enhanced cytotoxic effect of ara-c by low intensity ultrasound to hl-60 cells. Cancer Lett. 2000;149:189–194. doi: 10.1016/s0304-3835(99)00358-4. [DOI] [PubMed] [Google Scholar]
- 16.Saito K, Miyake K, McNeil PL, Kato K, Yago K, Sugai N. Plasma membrane disruption underlies injury of the corneal endothelium by ultrasound. Exp. Eye Res. 1999;68:421–427. doi: 10.1006/exer.1998.0626. [DOI] [PubMed] [Google Scholar]
- 17.Tachibana K, Tachibana S. Application of ultrasound energy as a new drug delivery system. Jpn. J. Appl. Phys. 1999;38:3014–3019. [Google Scholar]
- 18.Tachibana K, Uchida T, Ogawa K, yamashita N, Tamura K. Induction of cell-membrane porosity by ultrasound. Lancet. 1999;353:1409. doi: 10.1016/S0140-6736(99)01244-1. [DOI] [PubMed] [Google Scholar]
- 19.Cho C-W, Liu Y, Cobb WN, Henthorn TK, Lillehei K, Christians U, Ng K. Ultrasound-induced mild hyperthermia as a novel approach to increase drug uptake in brain microvessel endothelial cells. Pharm. Res. 2002;19:1123–1129. doi: 10.1023/a:1019837923906. [DOI] [PubMed] [Google Scholar]
- 20.Joshi A, Raje J. Sonicated transdermal drug transport. J Control Release. 2002;83:13–22. doi: 10.1016/s0168-3659(02)00200-6. [DOI] [PubMed] [Google Scholar]
- 21.Mitragotri S. Innovation - healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nature Reviews Drug Discovery. 2005;4:255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- 22.Pitt WG. Defining the role of ultrasound in drug delivery. Am J Drug Deliv. 2003;1:27–42. [Google Scholar]
- 23.Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery - a general review. Expert Opin Drug Delivery. 2004;1:37–56. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klibanov AL. Microbubbles for drug and gene delivery. Advanced Drug Delivery Reviews. 2008 doi: 10.1016/j.addr.2008.03.005. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang S. Liposomes in ultrasonic drug and gene delivery. Advanced Drug Delivery Reviews. 2008 doi: 10.1016/j.addr.2008.03.003. accepted. [DOI] [PubMed] [Google Scholar]
- 26.Apfel RE, Holland CK. Gauging the liklihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound. Ultrasound Med. Biol. 1991;17:179–185. doi: 10.1016/0301-5629(91)90125-g. [DOI] [PubMed] [Google Scholar]
- 27.Barnett S. Thresholds for nonthermal biofeffects: Theoretical and experimental basis for a threshold index. Ultrasound Med Biol. 1998;24:S41–S49. doi: 10.1016/s0301-5629(98)80001-x. [DOI] [PubMed] [Google Scholar]
- 28.Church CC. Frequency, pulse length, and the mechanical index. Acoustics Research Letters Online-Arlo. 2005;6:162–168. [Google Scholar]
- 29.Rangel-Yagui CO, Pessoa A, Tavares LC. Micellar solubilization of drugs. Journal of Pharmacy and Pharmaceutical Sciences. 2005;8:147–163. [PubMed] [Google Scholar]
- 30.Gaucher G, Dufresne MH, Sant VP, Kang N, Maysinger D, Leroux JC. Block copolymer micelles: Preparation, characterization and application in drug delivery. Journal of Controlled Release. 2005;109:169–188. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 31.Aliabadi HM, Lavasanifar A. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 2006;3:139–162. doi: 10.1517/17425247.3.1.139. [DOI] [PubMed] [Google Scholar]
- 32.Sarker DK. Engineering of nanoemulsions for drug delivery. Current Drug Delivery. 2005;2:297–310. doi: 10.2174/156720105774370267. [DOI] [PubMed] [Google Scholar]
- 33.Rawat M, Singh D, Saraf S, Saraf S. Nanocarriers: Promising vehicle for bioactive drugs. Biol. Pharm. Bull. 2006;29:1790–1798. doi: 10.1248/bpb.29.1790. [DOI] [PubMed] [Google Scholar]
- 34.Kohli P, Martin CR. Smart nanotubes for biotechnology. Current Pharmaceutical Biotechnology. 2005;6:35–47. doi: 10.2174/1389201053167211. [DOI] [PubMed] [Google Scholar]
- 35.Tiera MJ, Winnik FM, Fernandes JC. Synthetic and natural polycations for gene therapy: State of the art and new perspectives. Current Gene Therapy. 2006;6:59–71. doi: 10.2174/156652306775515510. [DOI] [PubMed] [Google Scholar]
- 36.Soma CE, Dubernet C, Bentolila D, Benita S, Couvreur P. Reversion of multidrug resistance by co-encapsulation of doxorubicin and cyclosporin a in polyalkylcyanoacrylate nanoparticles. Biomaterials. 2000;21:1–7. doi: 10.1016/s0142-9612(99)00125-8. [DOI] [PubMed] [Google Scholar]
- 37.Kabanov AV, Batrakova EV, Melik-Nubarov NS, Fedoseev NA, Dorodnich TY, Alakhov VY, Nazarova IR, Kabanov VA. A new class of drug carriers: Micelles of poly(oxyethylene)-poly(oxypropylene) block copolymers as microcontainers for targeting drugs from blood to brain. J. Controlled Release. 1992;22:141–158. [Google Scholar]
- 38.Rapoport NY, Marin AP, Timoshin AA. Effect of a polymeric surfactant on electron transport in hl-60 cells. Arch. Biochem. Biophys. 2000;384:100–108. doi: 10.1006/abbi.2000.2104. [DOI] [PubMed] [Google Scholar]
- 39.Rapoport NY, Herron JN, Pitt WG, Pitina L. Micellar delivery of doxorubicin and its paramagnetic analog, ruboxyl, to hl-60 cells: Effect of micelle structure and ultrasound on the intracellular drug uptake. J Controlled Rel. 1999;58:153–162. doi: 10.1016/s0168-3659(98)00149-7. [DOI] [PubMed] [Google Scholar]
- 40.Rapoport N, Pitt WG, Smirnov AI, Timoshin AI. Bioreduction of tempone and spin-labeled gentamicin by gram-negative bacteria: Kinetics and effect of ultrasound. Arch. Biochem. Biophys. 1999;362:233–241. doi: 10.1006/abbi.1998.1020. [DOI] [PubMed] [Google Scholar]
- 41.Howard B, Gao A, Lee S-W, Seo M-H, Rapoport N. Ultrasound-enhanced chemotherapy of drug-resistant breast cancer tumors by micellar-encapsulated paclitaxel. Am. J. Drug Deliv. 2006;4:97–104. [Google Scholar]
- 42.Bioactive polymer systems. In: Dorn K, Hoerpel G, Ringsdorf H, editors; Gebelein CG, Carraher JCE, editors. Polymeric anti-tumor agents on a molecular and cellular level. New York: Plenum; 1985. pp. 531–585. [Google Scholar]
- 43.Kataoka K, Kwon GS, Yokoyama M, Okano T, Sakurai Y. Block copolymer micelles as vehicles for drug delivery. J. Controlled Release. 1993;24:119–132. [Google Scholar]
- 44.Yokoyama M, Kwon GS, Naito M, Okano T, sakurai Y, Seto T, Kataoka K. Preparation of micelle-forming polymer-drug conjugates. Bioconj. Chem. 1992;3:295–301. doi: 10.1021/bc00016a007. [DOI] [PubMed] [Google Scholar]
- 45.Yokoyama M, Sugiyama T, Okano T, Sakurai Y, Naito M, Kataoka K. Analysis of micelle formation of an adriamicin-conjugated poly(ethylene)glicol-poly(aspartic acid) block copolymer by gel permeation chromatography. Pharmac. Res. 1993;10:895–899. doi: 10.1023/a:1018921513605. [DOI] [PubMed] [Google Scholar]
- 46.Yokoyama M, Okano T, Sakurai Y, Ekimoto H, Shibazaki C, Kataoka K. Toxicity and antitumor activity against solid tumors of micelle-forming polymeric anticancer drug and its extremely long circulation in blood. Cancer Res. 1991;51:3229–3236. [PubMed] [Google Scholar]
- 47.Kwon GS, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Enhanced tumor accumulation and prolonged circulation times of micelle-forming poly(ethylene oxide-co-aspartate) block copolymeradriamycin conjugates; 6th International Symposium on Recent Advantages in Drug Delivery Systems; 1993. [Google Scholar]
- 48.Pechar M, Ulbrich K, Subr V, Seymour LW, Schacht EH. Poly(ethylene glycol) multiblock copolymer as a carrier of anti-cancer drug doxorubicin. Bioconjugate Chem. 2000;11:131–139. doi: 10.1021/bc990092l. [DOI] [PubMed] [Google Scholar]
- 49.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem. Rev. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 50.Chung JE, Yokoyama M, Yamato M, Aohagi T, Sakurai Y, Okano T. Thermoresponsive drug delivery from polymeric micelles constructed using block copolymers of poly(n-isopropylacrylamide) and poly(butylmethacrylate) J. Control. Rel. 1999;62:115–127. doi: 10.1016/s0168-3659(99)00029-2. [DOI] [PubMed] [Google Scholar]
- 51.Yokoyama M, Okano T, Sakurai Y, Fukushima S, Okamoto K, Kataoka K. Selective delivery of adriamycin to a solid tumor using a polymeric micelle carrier system. J. Drug Targetting. 1999;7:171–186. doi: 10.3109/10611869909085500. [DOI] [PubMed] [Google Scholar]
- 52.Kwon GS, Suwa S, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Physical entrapment of adriamicin in ab block copolymer micelles. Pharm Res. 1995;12:192–195. doi: 10.1023/a:1016266523505. [DOI] [PubMed] [Google Scholar]
- 53.Kabanov AV, Alakhov VY. Pluronic block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Critical Reviews in Therapeutic Drug Carrier Systems. 2002;19:1–73. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]
- 54.Batrakova EV, Dorodnych TY, Klinskil EY, Kliushnenkova EN, Shemchukova OB, Goncharova ON, Arjakov SA, Alakov VY, Kabanov AV. Anthracycline antibiotics non-covalently incorporated into the block copolymer micelles: In vivo evaluation of anti-cancer activity. Br. J. Cancer. 1996;74:1545–1552. doi: 10.1038/bjc.1996.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rapoport N, Caldwell K. Structural transitions in micellar solutions of pluronic p-105 and their effect on the conformation of dissolved cytochrome c: An electron paramagnetic resonance investigation. Colloids and Surfaces B: Biointerfaces. 1994;3:217–228. [Google Scholar]
- 56.Husseini GA, Myrup GD, Pitt WG, Christensen DA, Rapoport NY. Factors affecting acoustically-triggered release of drugs from polymeric micelles. J. Controlled Release. 2000;69:43–52. doi: 10.1016/s0168-3659(00)00278-9. [DOI] [PubMed] [Google Scholar]
- 57.Husseini GA, El-Fayoumi RI, O'Neill KL, Rapoport NY, Pitt WG. DNA damage induced by micellar-delivered doxorubicin and ultrasound: Comet assay study. Cancer Letters. 2000;154:211–216. doi: 10.1016/s0304-3835(00)00399-2. [DOI] [PubMed] [Google Scholar]
- 58.Rapoport N, Pitina L. Intracellular distribution and intracellular dynamics of a spin-labeled analog of doxorubicin. J. Pharm. Sci. 1998;87:321–325. doi: 10.1021/js970271g. [DOI] [PubMed] [Google Scholar]
- 59.Husseini GA, Diaz MA, Richardson ES, Christensen DA, Pitt WG. The role of cavitation in acoustically activated drug delivery. J. Controlled Release. 2005;107:253–261. doi: 10.1016/j.jconrel.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Husseini GA, Diaz de la Rosa MA, Gabuji T, Zeng Y, Christensen DA, Pitt WG. Release of doxorubicin from unstabilized and stabilized micelles under the action of ultrasound. J. Nanosci. Nanotech. 2007;7:1–6. doi: 10.1166/jnn.2007.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pruitt JD, Husseini G, Rapoport N, Pitt WG. Stabilization of pluronic p-105 micelles with an interpenetrating network of n,n-diethylacrylamide. Macromolecules. 2000;33:9306–9309. [Google Scholar]
- 62.Zeng Y, Pitt WG. Poly(ethylene oxide)-b-poly(n-isopropylacrylamide) nanoparticles with crosslinked cores as drug carriers. J. Biomat. Sci. Polym. Ed. 2005;16:371–380. doi: 10.1163/1568562053654121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeng Y, Pitt WG. A polymeric micelle system with a hydrolysable segment for drug delivery. J. Biomater. Sci. Polymer Edn. 2006;17:591–604. doi: 10.1163/156856206776986297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parlitz U, Englisch V, Scheffczyk C, Lauterborn W. Bifurcation structure of bubble oscillators. Journal of the Acoustical Society of America. 1990;88:1061–1077. [Google Scholar]
- 65.Diaz-De-La-Rosa MA. High-frequency ultrasound drug delivery and cavitation M.S. thesis. Provo, UT: Brigham Young University; 2007. [Google Scholar]
- 66.Husseini GA, Rapoport NY, Christensen DA, Pruitt JD, Pitt WG. Kinetics of ultrasonic release of doxorubicin from pluronic p105 micelles. Coll Surf B: Biointerfaces. 2002;24:253–264. [Google Scholar]
- 67.Husseini GA, Abdel-Jabbar NM, Mjalli FS, Pitt WG. Modeling and sensitivity analysis of acoustic release of doxorubicin from unstabilized pluronic p105 using an artificial neural network model. Technology in Cancer Research & Treatment. 2007;6:49–56. doi: 10.1177/153303460700600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stevenson-Abouelnasr D, Husseini GA, Pitt WG. Further investigation of the mechanism of doxorubicin release from p105 micelles using kinetic models. Colloids and Surfaces B-Biointerfaces. 2007;55:59–66. doi: 10.1016/j.colsurfb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phelps AD, Leighton TG. The subharmonic oscillations and combination-frequency subharmonic emissions from a resonant bubble: Their properties and generation mechanisms. Acustica. 1997;83:59–66. [Google Scholar]
- 70.Fairbairn DW, Olive PL, O'Neill KL. The comet assay: A comprehensive review. Mutation Res. 1995;339:37–59. doi: 10.1016/0165-1110(94)00013-3. [DOI] [PubMed] [Google Scholar]
- 71.Husseini GA, O'Neill KL, Pitt WG. The comet assay to determine the mode of cell death for the ultrasonic delivery of doxorubicin to human leukemia (hl-60 cells) from pluronic p105 micelles. Technology in Cancer Research & Treatment. 2005;4:707–711. doi: 10.1177/153303460500400616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richardson ES, Pitt WG, Woodbury DJ. The role of cavitation in liposome formation. Biophysical Journal. 2007;93:4100–4107. doi: 10.1529/biophysj.107.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stringham SB, Murray BK, O'Neill KL, Ohmine S, Gaufin TA, Pitt WG. 96th Annual Meeting of the American Association for Cancer Research. Anaheim, CA, USA: AACR; 2005. Mechanism of targeted chemotherapeutic delivery using ultrasound. [Google Scholar]
- 74.Nelson JL, Roeder BL, Carmen JC, Roloff F, Pitt WG. Ultrasonically activated chemotherapeutic drug delivery in a rat model. Cancer Res. 2002;62:7280–7283. [PubMed] [Google Scholar]
- 75.Nelson JL. M.S. thesis. Provo, UT: Brigham Young University; 2002. Ultrasonically enhanced drug delivery of doxorubicin in vivo from stabilized pluronic micelle carriers. [Google Scholar]
- 76.Staples BJ. M.S. thesis. Provo, UT: Brigham Young University; 2007. Pharmacokinetics of ultrasonically-released, micelle-encapsulated doxorubicin in the rat model and its effect on tumor growth. [Google Scholar]
- 77.Rapoport N, Gao ZG, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. Journal of the National Cancer Institute. 2007;99:1095–1106. doi: 10.1093/jnci/djm043. [DOI] [PubMed] [Google Scholar]
- 78.Myhr G, Moan J. Synergistic and tumour selective effects of chemotherapy and ultrasound treatment. Cancer Letters. 2006;232:206–213. doi: 10.1016/j.canlet.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 79.Marin A, Sun H, Husseini GA, Pitt WG, Christensen DA, Rapoport NY. Drug delivery in pluronic micelles: Effect of high-frequency ultrasound on drug release from micelles and intracellular uptake. J. Controlled Rel. 2002;84:39–47. doi: 10.1016/s0168-3659(02)00262-6. [DOI] [PubMed] [Google Scholar]
- 80.Husseini GA, Runyan CM, Pitt WG. Investigating the mechanism of acoustically activated uptake of drugs from pluronic micelles. BMC Cancer. 2002;2 doi: 10.1186/1471-2407-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muniruzzaman MD, Marin A, Luo Y, Prestwich GD, Pitt WG, Husseini G, Rapoport NY. Intracellular uptake of pluronic copolymer: Effects of the aggregation state. Colloids and Surfaces B: Biointerfaces. 2002;25:233–241. [Google Scholar]
- 82.Gao Z, Fain HD, Rapoport N. Ultrasound-enhanced tumor targeting of polymeric micellar drug carriers. Molecular Pharmaceutics. 2004;1:317–330. doi: 10.1021/mp049958h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rapoport N. Combined cancer therapy by micellar-encapsulated drug and ultrasound. International Journal of Pharmaceutics. 2004;227:155–162. doi: 10.1016/j.ijpharm.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 84.Rapoport NY, Christensen DA, Fain HD, Barrows L, Gao Z. Ultrasound-triggered drug targeting of tumors in vitro and in vivo. Ultrasonics. 2004;42:943–950. doi: 10.1016/j.ultras.2004.01.087. [DOI] [PubMed] [Google Scholar]
- 85.Sheikov N, McDannold N, Jolesz F, Zhang YZ, Tam K, Hynynen K. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier. Ultrasound in Medicine and Biology. 2006;32:1399–1409. doi: 10.1016/j.ultrasmedbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 86.Prentice P, Cuschierp A, Dholakia K, Prausnitz M, Campbell P. Membrane disruption by optically controlled microbubble cavitation. Nature Physics. 2005;1:107–110. [Google Scholar]
- 87.Yamashita N, Tachibana K, Ogawa K, Tsujita N, Tomita A. Scanning electron microscopic evaluation of the skin surface after ultrasound exposure. Anatom. Rec. 1997;247:455–461. doi: 10.1002/(SICI)1097-0185(199704)247:4<455::AID-AR3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 88.Ogawa K, Tachibana K, Uchida T, Tai T, Yamashita N, Tsujita N, Miyauchi R. High-resolution scanning electron microscopic evaluation of cell-membrane porosity by ultrasound. Med Electron Microsc. 2001;34:249–253. doi: 10.1007/s007950100022. [DOI] [PubMed] [Google Scholar]
- 89.Miura S, Tachibana K, Okamoto T, Saku K. In vitro transfer of antisense oligodeoxynucleotides into coronary endothelial cells by ultrasound. Biochem Biophys Res Commun. 2002;298:587–590. doi: 10.1016/s0006-291x(02)02467-1. [DOI] [PubMed] [Google Scholar]
- 90.Taniyama Y, Tachibana K, Hiraoka K, Aoki M, Yamamoto S, Matsumoto K, Nakamura T, Ogihara T, Kaneda Y, Morishita R. Development of safe and efficient novel nonviral gene transfer using ultrasound: Enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Therapy. 2002;9:372–380. doi: 10.1038/sj.gt.3301678. [DOI] [PubMed] [Google Scholar]
- 91.Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K, Morishita R. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002;105:1233–1239. doi: 10.1161/hc1002.105228. [DOI] [PubMed] [Google Scholar]
- 92.Sivakumar M, Tachibana K, Pandit AB, Yasui K, Tuziuti T, Towata A, IIDA Y. Transdermal drug delivery using ultrasound-theory, understanding and critical analysis. Cellular and Molecular Biology. 2005;51 [PubMed] [Google Scholar]
- 93.Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC, Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene Ther. 2000;7:2023–2027. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- 94.Lawrie A, Brisken AF, Francis SE, Wyllie D, Kiss-Toth E, Qwarnstrom EE, Dower SK, Crossman DC, Newman CM. Ultrasound-enhanced transgene expression in vascular cells is not dependent upon cavitation-induced free radicals. Ultrasound Med Biol. 2003;29:1453–1461. doi: 10.1016/s0301-5629(03)01032-9. [DOI] [PubMed] [Google Scholar]
- 95.Lawrie A, Brisken AF, Francis SE, Tayler DI, Chamberlain J, Crossman DC, Cumberland DC, Newman CM. Ultrasound enhances reporter gene expression after transfection of vascular cells in vitro. Circulation. 1999;99:2617–2620. doi: 10.1161/01.cir.99.20.2617. [DOI] [PubMed] [Google Scholar]
- 96.Zuhorn IS, Engberts JBFN, Hoekstra D. Gene delivery by cationic lipid vectors: Overcoming cellular barriers. European Biophysics Journal with Biophysics Letters. 2007;36:349–362. doi: 10.1007/s00249-006-0092-4. [DOI] [PubMed] [Google Scholar]
- 97.Ma B, Zhang S, Jiang H, Zhao B, Lv H. Lipoplex morphologies and their influences on transfection efficiency in gene delivery. J Controlled Release. 2007;123:184–194. doi: 10.1016/j.jconrel.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 98.Li WJ, Szoka FC. Lipid-based nanoparticles for nucleic acid delivery. Pharmaceutical Research. 2007;24:438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 99.Kuo JHS, Jan MS, Sung KC. Evaluation of the stability of polymer-based plasmid DNA delivery systems after ultrasound exposure. International Journal of Pharmaceutics. 2003;257:75–84. doi: 10.1016/s0378-5173(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 100.Zobel HP, Werner D, Gilbert M, Noe CR, Stieneker F, Kreuter J, Zimmer A. Effect of ultrasonication on the stability of oligonucleotides adsorbed on nanoparticles and liposomes. J Microencapsul. 1999;16:501–509. doi: 10.1080/026520499288942. [DOI] [PubMed] [Google Scholar]
- 101.Rocha A, Ruiz S, Coll JM. Improvement of DNA transfection with cationic liposomes. J Physiol Biochem. 2002 Mar;58(1):45–56. doi: 10.1007/BF03179837. 58 (2002) 45–56. [DOI] [PubMed] [Google Scholar]
- 102.Hunter AC. Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Advanced Drug Delivery Reviews. 2006;58:1523–1531. doi: 10.1016/j.addr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 103.Zhou QY, Gallo JM. In vivo microdialysis for pk and pd studies of anticancer drugs. AAPS Journal. 2005;7:E659–E667. doi: 10.1208/aapsj070366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kichler A. Gene transfer with modified polyethylenimines. Journal of Gene Medicine. 2004;6:S3–S10. doi: 10.1002/jgm.507. [DOI] [PubMed] [Google Scholar]
- 105.Heyes J, Hall K, Tailor V, Lenz R, MacLachlan I. Synthesis and characterization of novel poly(ethylene glycol)-lipid conjugates suitable for use in drug delivery. Journal of Controlled Release. 2006;112:280–290. doi: 10.1016/j.jconrel.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 106.Hu FQ, Zhao MD, Yuan H, You J, Du YZ, Zeng S. A novel chitosan oligosaccharide-stearic acid micelles for gene delivery: Properties and in vitro transfection studies. International Journal of Pharmaceutics. 2006;315:158–166. doi: 10.1016/j.ijpharm.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 107.Benns JM, Kim SW. Tailoring new gene delivery designs for specific targets. Journal of Drug Targeting. 2000;8:1–12. doi: 10.3109/10611860009009205. [DOI] [PubMed] [Google Scholar]
- 108.Mansouri S, Cuie Y, Winnik F, Shi Q, Lavigne P, Benderdour M, Beaumont E, Fernandes JC. Characterization of folate-chitosan-DNA nanoparticles for gene therapy. Biomaterials. 2006;27:2060–2065. doi: 10.1016/j.biomaterials.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 109.Germershaus O, Merdan T, Bakowsky U, Behe M, Kissel T. Trastuzumab-polyethylenimine-polyethylene glycol conjugates for targeting her2-expressing tumors. Bioconjugate Chemistry. 2006;17:1190–1199. doi: 10.1021/bc0601119. [DOI] [PubMed] [Google Scholar]
- 110.Arote R, Kim TH, Kim YK, Hwang SK, Jiang HL, Song HH, Nah JW, Cho MH, Cho CS. A biodegradable poly(ester amine) based on polycaprolactone and polyethylenimine as a gene carrier. Biomaterials. 2007;28:735–744. doi: 10.1016/j.biomaterials.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 111.Unger EC, Hersh E, Vannan M, Matsunaga TO, McCreery T. Local drug and gene delivery through microbubbles. Prog Cardiovasc Dis. 2001;44:45–54. doi: 10.1053/pcad.2001.26443. [DOI] [PubMed] [Google Scholar]
- 112.Unger EC, Hersh E, Vannan M, McCreery T. Gene delivery using ultrasound contrast agents. Echocardiography. 2001;18:355–361. doi: 10.1046/j.1540-8175.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 113.Unger E, Porter T, Culp W, Labell R, Matsunaga TO, Zutshi R. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev. 2004;56:1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 114.Unger EC. Sonoporation and gene delivery with acoustically active carriers. European Journal of Ultrasound. 1997;6:1–2. [Google Scholar]
- 115.Lee JM, Takahashi M, Mon H, Koga K, Kawaguchi Y, Kusakabe T. Efficient gene transfer into silkworm larval tissues by a combination of sonoporation and lipofection. Cell Biology International. 2005;29:976–979. doi: 10.1016/j.cellbi.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 116.Mehier-Humbert S, Bettinger T, Yan F, Guy RH. Ultrasound-mediated gene delivery: Kinetics of plasmid internalization and gene expression. Journal of Controlled Release. 2005;104:203–211. doi: 10.1016/j.jconrel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 117.Mehier-Humbert S, Bettinger T, Yan F, Guy RH. Plasma membrane poration induced by ultrasound exposure: Implication for drug delivery. Journal of Controlled Release. 2005;104:213–222. doi: 10.1016/j.jconrel.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 118.Koch S, Pohl P, Cobet U, Rainov NG. Ultrasound enhancement of liposome-mediated cell transfection is caused by cavitation effects. Ultrasound Med Biol. 2000;26:897–903. doi: 10.1016/s0301-5629(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 119.Deng CX, Xu QH, Apfel RE, Holland CK. In vitro measurements of inertial cavitation thresholds in human blood. Ultrasound in Medicine and Biology. 1996;222:939–948. doi: 10.1016/0301-5629(96)00104-4. [DOI] [PubMed] [Google Scholar]
- 120.Deng CX, Xu QH, Apfel RE, Holland CK. Inertial cavitation produced by pulsed ultrasound in controlled host media. Journal of the Acoustical Society of America. 1996;100:1199–1208. doi: 10.1121/1.416304. [DOI] [PubMed] [Google Scholar]
- 121.Holland CK, Apfel RE. Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. Journal of the Acoustical Society of America. 1990;88:2059–2069. doi: 10.1121/1.400102. [DOI] [PubMed] [Google Scholar]
- 122.Larina V, Evers BM, Ashitkov TV, Bartels C, Larin KV, Esenaliev RO. Enhancement of drug delivery in tumors by using interaction of nanoparticles with ultrasound radiation. Technology in Cancer Research & Treatment. 2005;4:217–226. doi: 10.1177/153303460500400211. [DOI] [PubMed] [Google Scholar]
- 123.Larina IV, Evers BM, Bartels C, Ashitkov TV, Larin KV, Esenaliev RO. Ultrasound-enhanced drug delivery for efficient cancer therapy; Joint Meeting of the IEEE Engineering in Medicine and Biology Society And the Biomedical Engineering Society; Houston, TX. 2002. [Google Scholar]
- 124.Larina IV, Evers BM, Esenaliev RO. Optimal drug and gene delivery in cancer cells by ultrasound-induced cavitation. Anticancer Research. 2005;25:149–156. [PubMed] [Google Scholar]
- 125.Ivanoa Y, Evers BM, Thomas R, Ashitkov TV, Esenaliev RO. Nanoparticles and ultrasound for delivery of model macromolecular anti-cancer drugs in tumors; Joint Meeting of the IEEE Engineering in Medicine and Biology Society And the Biomedical Engineering Society; Houston, TX. 2002. [Google Scholar]
- 126.Zhou Q-H, Miller DL, Carlisle RC, Seymour LW, Oupicky D. Ultrasound-enhanced transfection activity of hpma-stabilized DNA polyplexes with prolonged plasma circulation. J. Control. Rel. 2005;106:416–427. doi: 10.1016/j.jconrel.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 127.Chumakova OV, Liopo AV, Andreev VG, Cicenaite I, Evers BM, Chakrabarty S, Pappas TC, Esenaliev RO. Composition of plga and pei/DNA nanoparticles improves ultrasound-mediated gene delivery in solid tumors in vivo. Cancer Letters. 2008 doi: 10.1016/j.canlet.2007.11.023. in press. [DOI] [PubMed] [Google Scholar]
- 128.Aoyama T, Hosseinkhani H, Yamamoto S, Ogawa O, Tabata Y. Enhanced expression of plasmid DNA-cationized gelatin complex by ultrasound in murine muscle. Journal of Controlled Release. 2002;80:345–356. doi: 10.1016/s0168-3659(02)00029-9. [DOI] [PubMed] [Google Scholar]
- 129.Hosseinkhani H, Aoyama T, Ogawa O, Tabata Y. Liver targeting of plasmid DNA by pullulan conjugation based on metal coordination. Journal of Controlled Release. 2002;83:287–302. doi: 10.1016/s0168-3659(02)00201-8. [DOI] [PubMed] [Google Scholar]
- 130.Hosseinkhani H, Aoyama T, Ogawa O, Tabata Y. Ultrasound enhancement of in vitro transfection of plasmid DNA by a cationized gelatin. Journal of Drug Targeting. 2002;10:193–204. doi: 10.1080/10611860290022624. [DOI] [PubMed] [Google Scholar]
- 131.Hosseinkhani H, Kushibiki T, Matsumoto K, Nakamura T, Tabata Y. Enhanced suppression of tumor growth using a combination of nk4 plasmid DNA-peg engrafted cationized dextran complex and ultrasound irradiation. Cancer Gene Therapy. 2006;13:479–489. doi: 10.1038/sj.cgt.7700918. [DOI] [PubMed] [Google Scholar]
- 132.Hosseinkhani H, Tabata Y. Ultrasound enhances in vivo tumor expression of plasmid DNA by peg-introduced cationized dextran. Journal of Controlled Release. 2005;108:540–556. doi: 10.1016/j.jconrel.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 133.Chen YC, Liang HD, Zhang QP, Blomley MJK, Lu QL. Pluronic block copolymers: Novel functions in ultrasound-mediated gene transfer and against cell damage. Ultrasound in Medicine and Biology. 2006;32:131–137. doi: 10.1016/j.ultrasmedbio.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 134.Unger EC, McCreery TP, Sweitzer RH, Caldwell VE, Wu Y. Acoustically active lipospheres containing paclitaxel: A new therapeutic ultrasound contrast agent. Invest Radiol. 1998;33:886–892. doi: 10.1097/00004424-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 135.Kabanov AV, Batrakova EV, Sherman S, Alakhov VY. Polymer genomics. 2006;193:173–198. [Google Scholar]
- 136.Nishiyama N, Kataoka K. Nanostructured devices based on block copolymer assemblies for drug delivery: Designing structures for enhanced drug function. Adv. Polymer Sci. 2006;193:67–101. [Google Scholar]
- 137.Satchi-Fainaro R, Duncan R, Barnes CM. Polymer therapeutics for cancer: Current status and future challenges. Adv Polym Sci. 2006;193:1–65. [Google Scholar]
- 138.Mitragotri S, Kost J. Low-frequency sonophoresis. A review. Adv Drug Deliv Rev. 2004;56:589–601. doi: 10.1016/j.addr.2003.10.024. [DOI] [PubMed] [Google Scholar]


