Abstract
Cells of the monocyte/macrophage lineage have been shown to be the principal targets for productive HIV-1 replication within the central nervous system. In addition, HIV-1-associated dementia (HAD) has been shown to correlate with macrophage abundance in the brain. While increased entry of monocytes into the brain is thought to initiate this process, mechanisms that prevent macrophage egress from the brain and means that prevent macrophage death may also contribute to cell accumulation. We hypothesized that osteopontin (OPN) was involved in the accumulation of macrophages in the brain in neuroAIDS. Utilizing in vitro model systems, we have demonstrated role of OPN in two distinct aspects of macrophage accumulation: prevention from recirculation, and protection from apoptosis. In these unique mechanisms, osteopontin would aid in macrophage survival and accumulation in the brain, the pathological substrate of HAD.
Introduction
A multitude of studies has shown that the primary cell population that serves as host for productive human immunodeficiency virus type 1 (HIV-1) replication within the central nervous system (CNS) consists of monocytic lineage cells, macrophage and microglia [1, 2]. The important roles that such cells play in HIV-1 infection strongly suggest that these cells are determinants in the progression and outcome of HIV-1-associated CNS diseases, collectively known as neuroAIDS.
Macrophages, which can produce numerous products potentially harmful to the CNS, are prime candidates in mediating indirect damage to the CNS initiated by HIV infection. Macrophages in brain and other tissues originate from circulating blood monocytes, which in turn are generated from precursor cells in the bone marrow. In addition to the predominant population of blood monocytes (CD14+CD16-), a subset of monocytes also expresses CD16 (Fcγreceptor III). In HIV-1 associated dementia (HAD), an increase in this subset (CD14+CD16+) of monocytes is found in the blood [3]. In the brain during HIV-1 encephalitis (HIVE), cells of a similar phenotype accumulate in perivascular locations (known as perivascular macrophages), and are often infected with HIV-1. Whether the CD16+ monocytes in the blood preferentially become CD16+ macrophages in tissue is not known. However, recent data reveal that such cells have a high ability to migrate across an endothelial cell layer, as well as reverse transmigrate from an ablumenal to lumenal location [4]. This monocyte subset is also intriguing in regards to neuropathogenesis as it can produce high levels of the pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) [5-7], which may play a major role in neuronal death. Both TNF-α and IL-1 increase the permeability of the blood-brain barrier (BBB), subsequently allowing additional HIV-infected monocytes to enter the brain [8]. Moreover, TNF-α up-regulates the expression and release of various chemokines in the CNS, such as monocyte chemoattractant protein 1(MCP-1/CCL2), a potent chemoattractant for monocytes [8, 9].
Recent studies have shown that CD16+ monocytes express high levels of CX3CR1, the receptor for fractalkine/CX3CL1, and low levels of CCR2, a receptor for CCL2 [10-12]. These cells show efficient transendothelial migration in response to CX3CL1, but not CCL2 [10, 11]. These studies identified CX3CL1 as a major chemokine and adhesion molecule mediating CD16+ monocyte arrest and migration. However it has also been shown that increased CCL2 expression in the cerebrospinal fluid (CSF) occurs in conjunction with HAD and HIVE. In vitro studies have revealed that CCL2 can induce the translocation of monocytes across a model of the BBB [13-15]. Although likely contributing to an increase in monocyte entry into the brain, the relative role of these chemokines and other molecules in the macrophage accumulation that occurs In addition to chemokine-induced monocyte ingress, a normal physiological trafficking of monocytes into the brain occurs. Further unknown mechanisms may also participate in driving blood monocytes into the brain. Still, additional means likely contribute to the increased number ofmacrophages in the brain, such as mechanisms that prevent macrophage egress and means that prevent monocyte/macrophage death. Although monocyte entry into the brain is the subject of much focus, mechanisms that retain macrophages in the brain are under explored.
Osteopontin (OPN) is an extracellular protein involved in differentiation and immune cell activation as well as cell attachment and migration [16]. OPN has two receptors on monocytes/macrophages and other cellular targets: CD44 variant 6 (CD44v6) and certain integrins, most notably the α(V) and β(1) integrins, which also play crucial roles in monocyte transmigration [17]. CD44v6 mediates macrophage chemotactic migration in response to osteopontin [18], as well as playing an important role in monocytes differentiating to macrophages [19]. In addition to its involvement in migration, OPN prevents the apoptosis of endothelial cells, melanocytes, renal epithelial cells, and IL-3-dependent hematopoietic cells [16, 20-22].
We have recently found that expression of osteopontin was increased in the brains of monkeys with simian immunodeficiency virus (SIV) encephalitis (SIVE), a nonhuman primate model of HIV-induced brain disease [23]. Here, we report that OPN is also increased in the plasma and CSF of animals with SIVE. We hypothesized that OPN was involved in the accumulation of macrophages in the brain in HIV induced CNS disease, and here have analyzed the role of OPN in three distinct aspects of monocyte/macrophage biology: chemotaxis, prevention from recirculation, and protection from apoptosis. In these ways, OPN could aid in monocyte/macrophage infiltration and accumulation in the brain, the pathological substrate of HIV associated dementia.
Materials and Methods
Rhesus macaques
Rhesus monkeys were infected with serially-passaged derivatives of SIVmac251 [24, 25]. Animals were categorized as: SIV encephalitis (SIVE+, n=12; rhesus 383, 494, 418, 427, 296, 260, 289, 301, 321, 323, 328, and 330) and SIV without encephalitis (SIVnoE, n=8; rhesus 265, 231, 322, 403, 492, 424, 422 and 360). SIV encephalitis was diagnosed by the presence of multinucleated giant cells, microglial nodules and infiltration of macrophages.
Rhesus monkey plasma and cerebral spinal fluid (CSF) collection
Rhesus macaques had plasma and CSF samples collected under ketamine anesthesia. For plasma, blood was placed in EDTA-treated tubes and centrifuged for separation from cells and erythrocytes. CSF samples were also centrifuged for elimination of cells.
Osteopontin measurement
The levels of osteopontin in plasma and CSF at the time of necropsy from monkeys were measured using a human osteopontin enzyme-linked immunosorbence assay (ELISA) (Immuno-Biological Laboratories, IBL-America; Minneapolis, MN). Samples were analyzed in duplicate according to the manufacturer’s instructions. Plasma and CSF (n=30) samples were also analyzed from different animals prior to infection to establish baseline OPN levels.
Isolation of peripheral blood mononuclear cells (PBMCs) and purification of human monocytes
Normal human blood was obtained from The Scripps Research Institute Normal Blood Donor Service (NBD; La Jolla, CA) into EDTA-containing syringe (1 ml of 7.4% EDTA per 60 ml blood drawn). To qualify for the NBD pool, volunteers were between 18 and 65 years of age, weighed at least 110 pounds (50 kg), had blood pressure between 90-180 mm Hg systolic and 50-100 mm Hg diastolic, have not had major surgery within the past six months, had an adequate hematocrit on recent testing, and had negative screening for HIV and Hepatitis B, and C at initial screening, and annually thereafter. Blood was processed using OptiPrep (OptiPrep™, Cell Separation Media, max. density=1.320 g/ml., max. osmolality=170 mOsm, Accurate Chemical, Westbury, NY) utilizing the manufacturers recommendations for enhancement of monocytes but scaled up for isolation of more blood. The monocyte-enriched PBMCs were then washed with RPMI with 2% FCS and pelleted and platelets were removed, and cells quantified using the Z2 series Coulter Counter (Beckman Coulter, Fullerton, CA). The resulting cell suspension contained between 40-60% monocytes (determined by CD14 APC FACS staining) and the remaining cells were mostly T and B cells. For some experiments, we utilized these monocyte-enriched PBMC, but for others we proceeded to isolate pure monocytes. Monocytes were isolated from the enriched PBMC utilizing the MACS Human Monocyte Isolation Kit II (Miltenyi Biotec, Auburn, CA) using the manufacturer’s instructions. Monocytes were counted and assayed for purity by FACS utilizing the mouse anti-human CD14 APC antibody (Beckman Coulter/Immunotech) (purity >95%).
Chemotaxis assay
Monocyte-enriched PBMCs, (using the OptiPrep method above), were utilized for the chemotaxis assays. Cells were resuspended at 106 cells per ml of RPMI and 3 mls were added to the top of each 6-well transwell (Corning; 3 μm pore size). Below the insert, 3 mls of RPMI containing the indicated concentration of chemokine (CCL2, CX3CL1, or OPN, all purchased from R & D Systems, Minneapolis, MN) was added. The cells were allowed to migrate for 3 hours (37° C, 5% CO2). Cells were collected from the bottom chamber, counted and stained with CD14 APC and mouse anti-human CD16 FITC antibody (BD Pharmingen) and examined by FACS. The migration indices were measured as fold over control (no chemokine) migration.
Preparation of collagen and HUVECs
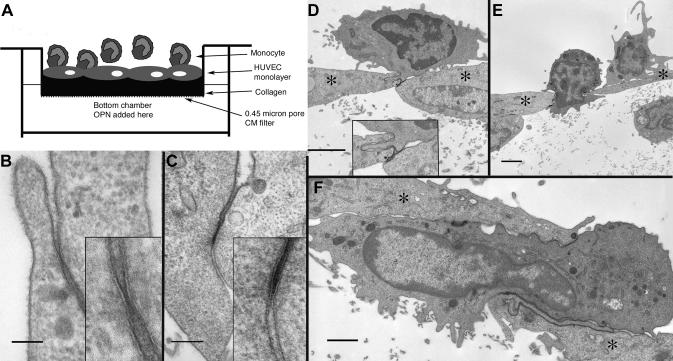
Millicell 6-well CM (hydrophilic PTFE) cell culture inserts (Millipore, Billerica, MA) with a 0.4-micron pore size were used. Monomeric collagen (Cohesion Vitrogen 100; Invitrogen, Palo Alto, CA) was prepared as recommended (8X Vitrogen, 1X 10X PBS and 1X 0.1 N NaOH, to a final pH 7.0) and 1.5 mls of collagen was added to the top of the CM filters and allowed to polymerize at 37° C for 1.5 hrs. Gels were coated with 1 ml of fibronectin at 37° C for 15 mins (Invitrogen/Gibco; 50 μg/ml in normal saline). Human umbilical vein endothelial cell (HUVEC) (Cascade Biologics, Portland, OR) monolayers were plated at 250,000 cells per well (50% confluency) and grown in Medium 200 with low serum growth supplement (Cascade Biologics) on the collagen for 2-3 days until confluent (see Figure 2A for schematic).
Figure 2. In vitro model for the examination of the reverse transmigration of human monocytes.
(A) Diagram of the in vitro filter model to study reverse transmigration. (B & C) Tight junctions between adjacent HUVECs; calibration bars: 100 nm & 200 nm respectively (Individual insets show enlargements of the junctions). (D - F) Three figures illustrating monocytes in transit through the layer of endothelial cells (*) into the collagen matrix; calibration bars: 2 μm, 2 μm & 1 μm respectively.
Reverse Transmigration Assay
The reverse transmigration assay was described previously [4, 26-29]. Purified monocytes were resuspended at 106 cells/ml in MEM (Invitrogen/Gibco) containing 1 mg/ml human serum albumin (Sigma-Aldrich; St. Louis, MO). Medium overlying the confluent HUVECs was aspirated and 3 mls of the monocyte suspension was added and incubated at 37° C for 1.5 hours. The bottom chamber at this point contained 3 mls of MEM containing 1 mg/ml human serum albumin. After 1.5 hrs, cells on top of the collagen (prepared as above) were removed by aspirating the media and the collagen washed twice with 1 ml of Hanks buffer saline (HBSS, Gibco) containing 1 mM EGTA. The media underneath the filter was removed and 3 mls of new MEM only or MEM containing human recombinant osteopontin (750 ng/ml) was added. Two mls of 20% heat-inactivated human serum/MEM were added on top of the collagen and cultured for two days. Reverse transmigrated cells were collected from the layer of human serum/MEM, without touching endothelial monolayer and then the collagen was washed with ice cold HBSS containing 1mM EGTA. Cells were pelleted, counted using a hemacytometer and then stained for FACS for CD14 and CD16 cell populations. To digest collagen gels containing the HUVEC monolayer and non-reverse transmigrated cells, 3 mls per well of collagenase (Roche, Indianapolis, IN) 2 mg/ml in MEM (no serum added)) were added and allowed to digest the collagen for 45 mins at 37° C. The mixture was then strained though a 70 μm cell strainer. Cells were pelleted, counted and stained for FACS as above. The percent of reverse transmigrated cells was calculated as the number of reverse transmigrated monocytes divided by the sum of the reverse transmigrated monocytes and number of cells left behind in the collagen multiplied by 100.
Electron microscopy
The same procedure was used to setting up the collagen and migration/reverse migration procedure, but was scaled down to use the CM filters in 24-well plates. Samples were fixed for EM based on a standard protocol [30]. Following the designated time interval for experimental treatment(s), the culture medium was removed and the entire filter complex fixed in 2.5% glutaraldehyde in 0.1 M Na cacodylate buffer (pH 7.3), washed in buffer, and then fixed in 1% osmium tetroxide in 0.1 M Na cacodylate buffer. The intact filters were subsequently treated with 0.5% tannic acid followed by 1% sodium sulfate, washed in buffer, and then dehydrated in a graded ethanol series. During the initial 50% ethanol step, the filter holders were inverted and the filter with its associated sandwich of cells and collagen was carefully removed from the plastic frame by cutting around the perimeter of the frame base using a #11 scalpel blade. The intact filter ‘sandwich’ was fully dehydrated, cleared in propylene oxide and infiltrated overnight with Epon / Araldite resin (Electron Microscopy Sciences, Hatfield, PA). The circular filters with intact sample layers still on top were then sliced into long strips with a #11 scalpel blade and polymerized overnight in embedding molds. Semi-thick (1 - 2 μm) sections were stained with toluidine blue for general assessment in the light microscope. Thin sections (70 nm) were cut on a Reichert Ultracut E (Leica, Deerfield, IL) using a diamond knife (Diatome, Electron Microscopy Sciences, Hatfield PA), mounted on parlodion coated, copper, slot grids and stained in uranyl acetate and lead citrate. Sections were examined on a Philips CM100 TEM (FEI, Hillsbrough, OR) and data documented on Kodak SO-163 film for later analysis. Negatives were scanned at 600 lpi using a Fuji FineScan 2750×l (Enovation Graphics, Chicago IL) and converted to tiff format for subsequent handling in Adobe Photoshop.
Apoptosis assay and FACS
Pure monocytes were grown in serum-free RPMI media at 106 cells/ml under non-adherent conditions in VueLife (FEP) bags (American Fluoroseal Corporation, AFC; Gaithersburg, MD) for 24 hours (37° C, 5 % CO2), with or without the addition of recombinant human osteopontin (750 ng/ml). Control protein (human serum albumin, 750 ng/ml) was also used in some experiments for the cultures without osteopontin, and yielded results indistinguishable from those without albumin. After 24 hours cells were harvested and stained by FACS using the Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences; Franklin Lakes, NJ). The percents of alive (Annexin V-FITC neg. and PI neg.) and apoptotic (Annexin V-FITC pos. and PI pos.) cells were measured. In addition, monocytes were also stained with mouse anti-human CD44v6 biotin conjugated monoclonal antibody (Invitrogen/Biosource) and mouse anti-human integrin αVβ3 biotin conjugated monoclonal antibody (Chemicon), followed by streptavidin-allophycocyanin (SAv-APC) conjugate (BD Pharmingen), and the geometric mean fluorescent intensities were measured by FACS. The fluorescent intensity measurements were calibrated by the use of isotype control (Mouse IgG1 biotin control). Cells were acquired on a FACS Calibur using a zero threshold for cell size.
Statistical analysis
Statistical analyses used the Prism 4 software (GraphPad Software, Inc., San Diego, CA) and Delta Graph (Red Rock Software, Salt Lake City, UT).
Results
In order to extend prior DNA array results indicating the upregulation of osteopontin (OPN) in the brains of monkeys with simian immunodeficiency virus (SIV) encephalitis (SIVE) [23], we assayed plasma and CSF OPN levels in the rhesus macaque model. We analyzed plasma and CSF OPN levels at the time of necropsy in SIV-infected monkeys with SIV encephalitis (SIVE+), and without SIV encephalitis (SIVnoE) (Table 1). We also examined the plasma and CSF of 30 uninfected animals to establish baseline levels of OPN. These data reveal that the level of OPN found in the plasma during SIVE is significantly increased compared that found in SIVnoE (3.7-fold, Tukey’s Multiple comparison test p< 0.001) and uninfected animals (7.9-fold, Tukey’s Multiple comparison test p<0.001) (Table 1). In addition, the CSF OPN level in SIVE+ animals is significantly increased compared that found in SIVnoE (2.9-fold, Tukey’s Multiple comparison test p<0.01) and uninfected animals (12.8-fold, Tukey’s Multiple comparison test p<0.0001) (Table 1). Thus, OPN is upregulated in both the plasma and CSF during encephalitis in the rhesus model of neuroAIDS.
Table 1.
Osteopontin levels in the plasma and CSF of rhesus monkeys. In the SIV infected groups (SIV noE and SIVE+) plasma and CSF OPN levels were assayed from samples taken just before necropsy. The average OPN levels were are shown as well as the standard error of the mean and the number of rhesus monkeys utilized (N). The three groups of animals were significantly different when compared by ANOVA (plasma: F= 27.08, p<0.0001; CSF: F=16.41, p<0.0001)
| Group | OPN Plasma | OPN CSF | N |
|---|---|---|---|
| Uninfected | 76.2 ± 7.4 ng/ml | 97.0 ± 18.1 ng/ml | 30 |
| SIVnoE | 164.1 ± 23.5 ng/ml | 422.6 ± 80.2 ng/ml | 8 |
| SIVE+ | 600.1 ± 122.5 ng/ml | 1244.4 ± 337.1 ng/ml | 12 |
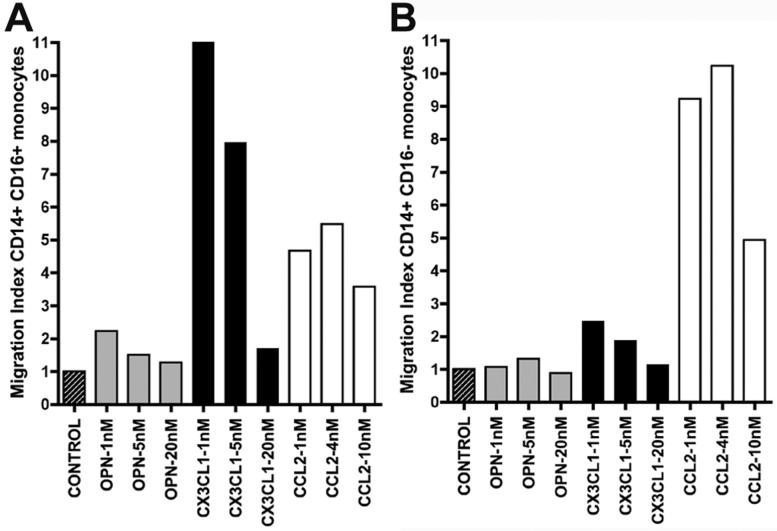
Next, we assessed the ability of OPN, CCL2, and CX3CL1 to induce human monocyte migration. Data were expressed as migration indices, calculated by dividing the percentage of migration obtained in the presence of chemokine by the percentage of migration for negative controls (Figure 1A-B). CD14+CD16+ monocytes were attracted to CX3CL1 (Figure 1A), and CD14+CD16- monocytes were induced to migrate with CCL2 (Figure 1B). However, OPN did not affect the migration of CD14+CD16+ monocytes (Figure 1A) or CD14+CD16- monocytes (Figure 1B), as compared to control. Thus, we concluded that in this model OPN does not behave as a classic chemokine to monocytes.
Figure 1. OPN is not a classic chemokine for monocytes.
Control is shown in slashed boxes, OPN in grey, CX3CL1 (fractalkine) in black and CCL2 in white. Values are relative to control and shown is one representative experiment out of three performed. (A) Shown are the CD14+CD16+ monocyte migration indices. CX3CL1 is known to be a potent chemoattractant for CD14+CD16+ monocytes. All OPN indices shown are similar to control. (B) Shown are the CD14+CD16- monocyte migration indices. CCL2 is known to be a potent chemoattractant for CD14+CD16- monocytes. All OPN indices shown are similar to control. 1, 5, and 20 nM of OPN corresponds to concentrations of 65, 325, and 1300 ng/ml; 1, 5, and 20 nM of CX3CL1 corresponds to concentrations of 90, 450, and 1800 ng/ml; 1, 4, and 20 nM of CCL2 corresponds to concentrations of 8.7, 35, and 87 ng/ml.
Under the hypothesis that OPN does not directly influence the accumulation of macrophages in the brain as a chemokine, we sought to examine other potential mechanisms, for instance, the ability of OPN in preventing macrophages from leaving the brain to recirculate in the body. Macrophage accumulation and retention in the brain were investigated using a model system of endothelial cells grown on a collagen gel (Figure 2A), in which monocytes can migrate beneath the endothelial cell layer as well as reverse transmigrate back; modified from a characterized model [4, 26-29] (see Material and Methods). Monocytes were added to the top (endothelial surface) layer, and allowed to transmigrate for 90 minutes at which time approximately 70% of the monocytes migrated across the monolayer into the collagen. Examination by electron microscopy demonstrated a confluent layer of endothelial cells, which possessed both tight and gap junctions between continuous cells (Figure 2B & C) as well as indications of basal lamina formation. Monocytes were readily visible both above and below the HUVEC layer. More importantly, monocytes were found in the process of migrating between endothelial cells of this confluent layer (Figures 2D - F).
Following the 90-minute monocyte migration, cultures then were extensively washed. Media was replaced, and OPN (at 750 ng/ml) added to the top or bottom chamber. Cultures were maintained for an additional 2 days to allow for reverse transmigration (monocytes moving from the ablumenal, i.e. tissue, side to the luminal, i.e. blood, side) of the in vitro endothelial monolayer. Cells on both sides of the endothelial layer were recovered, quantified, and phenotyped for CD14 and CD16 expression.
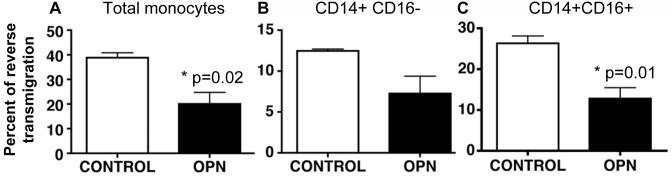
In control wells, an average of 39% of total monocytes reverse migrated, whereas in the presence of OPN in the bottom chamber 20% of cells reverse transmigrated (Figure 3A). Thus, OPN significantly decreased the percentage of cells that re-crossed the endothelial layer compared to control (Figure 3A; Student’s t test p= 0.02). Although reverse transmigration of both the CD14+CD16- and CD14+CD16+ subsets of monocytes appeared to decline, only the inflammatory CD14+CD16+ subset reached statistical significance, with a 51% reduction (Figure 3C; Student’s t test p= 0.01). Thus, more monocytes were trapped in the collagen when OPN was added underneath the collagen. When OPN was added to the top chamber (above the endothelial layer), no difference between control and OPN was found (data not shown).
Figure 3. Osteopontin decreased the percent of monocytes that reverse transmigrated.
(A) Osteopontin (750 ng/ml) significantly decreased the percent of total monocytes that reverse transmigrated compared to control (Student’s t test, p=0.02). (B) There is a decrease (however, non-significant p>0.05) in the percent of CD14+CD16- monocytes that reverse transmigrated compared to control. (C) A significant decrease in the amount of CD14+CD16+ monocytes that reverse transmigrated was demonstrated between OPN and control (Student’s t test p=0.01). The mean of 3 experiments with standard error of the mean is shown.
In addition to preventing reverse transmigration, we also hypothesized that osteopontin may play a role in another potential mechanism, preventing monocyte death. Therefore, we investigated whether OPN protects from the rapid apoptosis induced by culturing monocytes under serum deprivation [31-34]. In eight separate experiments using cells from different donors, human monocytes were cultured in non-adherent conditions in RPMI, plus and minus OPN (again at 750 ng/ml) (Figure 4A). After 24 hrs in culture the cells were stained with propidium iodide (PI) and Annexin V and analyzed by FACS (Figure 5A and B). The percentage of cells that were alive was determined as negative for both PI and Annexin V. The average percent of cells alive in the cultures treated with OPN (26%) was highly increased compared to those cultured in RPMI alone (average 6.6%) (Student’s t test p= 0.0005; Figure 4A and B). The average increase of OPN compared to RPMI was 4.7-fold (Figure 4B). Thus, OPN protects monocytes from apoptosis induced by serum deprivation.
Figure 4. Osteopontin protects monocytes from apoptosis.
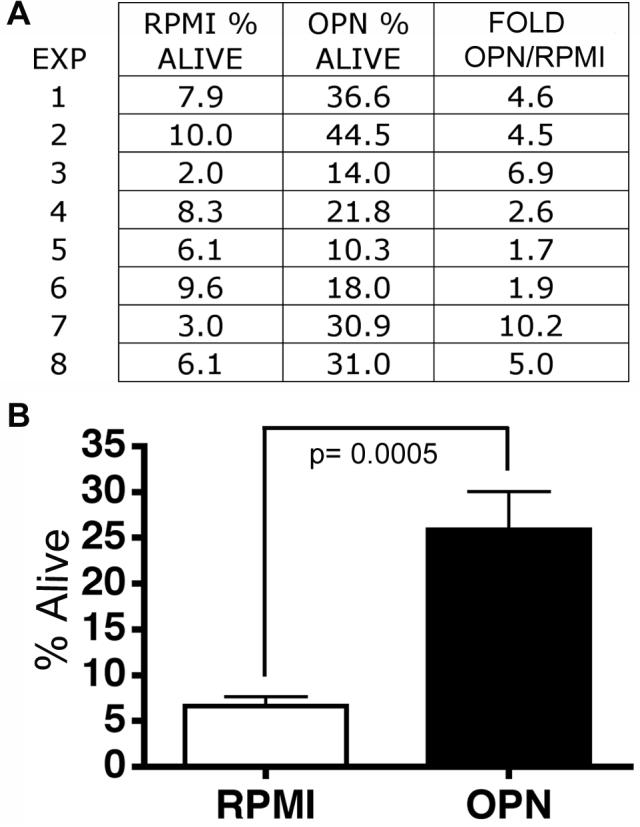
(A) In eight separate experiments (EXP 1-8), human monocytes were grown in non-adherent culture conditions for 24 hours. Shown is the percent of living cells (Annexin V and PI neg. cell population) in RPMI or cultured with 750 ng/ml of osteopontin (OPN). The fold is the increase in OPN compared to RPMI only. (B) The averages and standard error of the means are shown. OPN significantly increased the percentage of viable monocytes (4.7-fold, p= 0.0005).
Figure 5. FACS staining showed OPN decreased the percent of monocyte apoptosis.
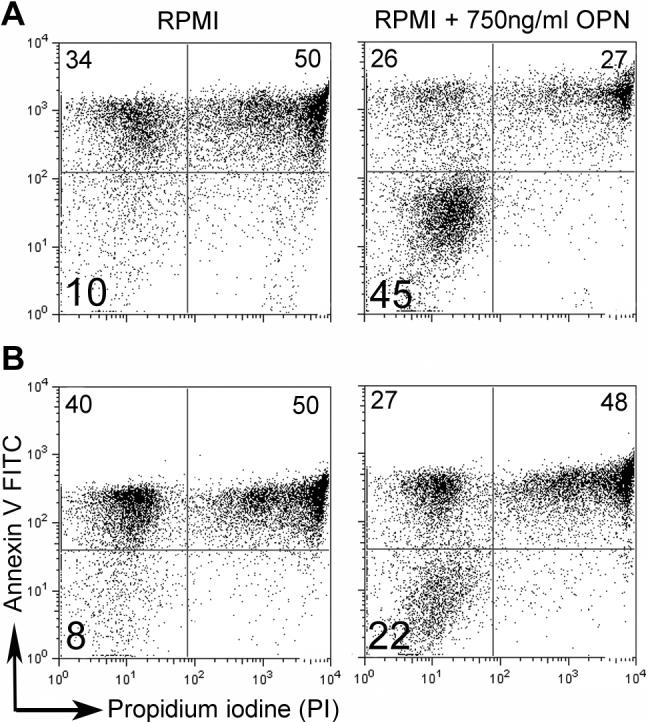
Annexin V-FITC and PI staining of two representative patients are shown (A and B). The left panel shows the cells cultured in RPMI for 24 hrs, and the right panel shows cells that were cultured in RPMI with 750 ng/ml of OPN for 24 hrs. Note that OPN increases live cell percentages on the lower left quadrant compared to control.
The expression of the OPN receptors, CD44v6 and integrin αVβ3, was also examined on the monocytes after 24 hrs in culture in either RPMI or with OPN (Figure 6A and B). Both CD44v6 and integrin αVβ3 expression were consistently increased when monocytes were cultured with OPN, although levels were variable between individuals.
Figure 6. OPN increases CD44v6 and integrin expression.
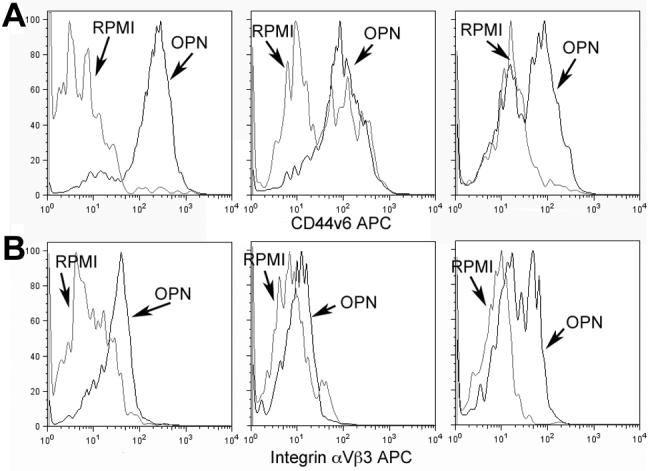
Arrows indicate the histograms for cells cultured in RPMI media (grey line) and OPN (black line). (A) CD44v6 expression in monocytes that were gated for Annexin V neg and PI neg (alive) cells after 24 hrs of culture is shown in three patients. (B) Integrin αVβ3 in monocytes that were gated for Annexin V neg and PI neg (alive) cells after 24 hrs of culture is shown in three patients.
Discussion
Our results demonstrate that OPN is upregulated in both the plasma and CSF of rhesus monkeys with SIVE, an animal model of HIV-induced brain disease. In addition, we have shown that although OPN is not a classic chemokine for monocytes, it does decrease reverse transmigration of monocytes (specifically the CD14+CD16+ subpopulation) and protects monocytes from apoptosis. Both of these mechanisms will result in an accumulation of macrophages in the brain. Since an increase expression of OPN in the brain, plasma and CSF is associated with SIVE, we conclude that OPN may be aiding in the accumulation of macrophages in the brain and thus neuropathology.
A normal physiological trafficking of monocytes into the brain occurs to replenish perivascular macrophages, which turnover on a regular basis [35]. Monocytes can also enter the brain through chemokine-induced entry during inflammation, such as following CCL2 or CX3CL1 expression [14]. Although monocyte entry into the brain is the subject of much study [1, 35], mechanisms that maintain macrophages in the brain themselves can also lead to macrophage accumulation, as increased entry is only one side of the equation in increased cell number. Here, we have shown two additional mechanisms of macrophage accumulation: prevention of monocyte/macrophage egress from the brain and prevention of monocyte death.
In normal conditions, monocytes continually enter the brain to become perivascular macrophages, however the fate of the older perivascular macrophages is unknown. They may senesce, although apoptosis has not been observed [36, 37], or they may become parenchymal microglia, which is likely a rare event [38]. The perivascular macrophages may re-enter the bloodstream or traverse the pial surface to enter lymphatics, however examining these possibilities have been experimentally challenging. Regardless, preventing re-entry or death, as we have shown here with OPN, will lead to cell accumulation.
Many studies have examined chemokine-induced migration of monocytes across vessels. Although many studies in neuroAIDS have focused upon CCL2, CCL2 is not effective in inducing transmigration of the CD16+ monocyte subset that has been linked to neuroAIDS, whereas CX3CL1 is capable of inducing their transendothelial migration, but has not been extensively examined in this disease [11, 18]. Although several studies suggest that OPN facilitates monocyte infiltration during infection or injury [41-43], we have not been able to confirm this in our model. Our findings that OPN induces a decrease in monocyte reverse transmigration and protects monocytes from apoptosis provide mechanisms by which OPN expression can lead to macrophage accumulation. This may be the case in conditions in which OPN is increased, such as in neuroAIDS.
Interestingly, an intracellular form of OPN has been described, which, if absent, impairs macrophage chemotaxis when chemokines working through G-protein coupled receptors are employed [44]. Here, extracellular OPN was added, limiting the exploration of this possibility. Thus, it remains possible that during neuroAIDS, monocytes in the periphery may have higher levels of intracellular OPN, which primes them to more readily enter and take residence in the brain.
Recent studies have shown that basal-to-apical transendothelial migration is dependent on p-glycoprotein (MDR-1) [28]. Antibodies to MDR-1 were identified as potent inhibitors of reverse transmigration of mononuclear phagocytes in an in vitro model of a vessel wall. However, the exact mechanism by which MDR-1 acts to facilitate migration remains unclear. Here, we have demonstrated another means of altering reverse transmigration of macrophages through OPN. OPN may utilize adhesion properties through interaction with its receptor CD44v6, which is found on macrophages, to trap cells in tissues.
While this manuscript was in review, a study was published online which showed OPN promoted the survival of activated mouse T cells, and linked increased OPN to disease progression in models of multiple sclerosis [45]. That study used 2,000 □g/ml or 10,000 □g/ml of OPN to demonstrate survival of T cells, whereas here we utilized 750 ng/ml, similar to the levels of OPN we found in the plasma during SIVE (600.1 ± 122.5 ng/ml), to demonstrate that OPN promotes the survival of human monocytes. Interestingly, CSF levels of OPN during SIVE are higher (1244.4 ± 337.1 ng/ml). It will be important in future studies to determine the levels of OPN that immune and other cells are exposed to in the brain itself.
We have also found that OPN increased the survival of monocytes. It is known that both soluble and extracellular matrix immobilized OPN can protect against apoptosis, but acting through different receptors and mechanisms [16, 46]. Since the anti-apoptotic effect of soluble OPN in IL-3-dependent hematopoietic cells was shown to be mediated through its interaction with the CD44 receptor and activation of the PI3K/Akt signaling pathway [16], this is a candidate mechanism.
The role of OPN in both decreasing the amount of macrophages returning to circulation and increasing the survival of those cells can lead to the accumulation of macrophages in the brain. Thus, OPN provides a strong amplifying force in brain macrophage infiltration and accumulation, the pathological substrate of neuroAIDS. The properties of OPN described here provide a novel explanation for accumulation of macrophages in the brain during neuroAIDS and other diseases such as multiple sclerosis. In order to prevent this accumulation and further damage caused by activated macrophages, therapeutic strategies may focus on the interfering with the production or actions of OPN.
Acknowledgements
These studies were supported by NIH grants MH62261, NS045534, and MH073490 (awarded to HSF). THB was supported by NRSA post-doctoral fellowship F32 NS048830. We thank Claudia Flynn, Jason Lee, Ryan Ojakian, Debbie Watry, and Michelle Zandonatti for technical assistance. This is manuscript #18606 from The Scripps Research Institute.
References
- 1.Kim WK, Corey S, Alvarez X, Williams K. Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol. 2003;74:650–6. doi: 10.1189/jlb.0503207. [DOI] [PubMed] [Google Scholar]
- 2.Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukoc Biol. 2003;74:691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- 3.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–5. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 4.Randolph GJ, Sanchez-Schmitz G, Liebman RM, Schakel K. The CD16(+) (FcgammaRIII(+)) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J Exp Med. 2002;196:517–27. doi: 10.1084/jem.20011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–42. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 6.Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–24. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- 7.Ancuta P, Weiss L, Haeffner-Cavaillon N. CD14+CD16++ cells derived in vitro from peripheral blood monocytes exhibit phenotypic and functional dendritic cell-like characteristics. Eur J Immunol. 2000;30:1872–83. doi: 10.1002/1521-4141(200007)30:7<1872::AID-IMMU1872>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Brabers NA, Nottet HS. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur J Clin Invest. 2006;36:447–58. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 9.Hurwitz AA, Lyman WD, Berman JW. Tumor necrosis factor alpha and transforming growth factor beta upregulate astrocyte expression of monocyte chemoattractant protein-1. J Neuroimmunol. 1995;57:193–8. doi: 10.1016/0165-5728(95)00011-p. [DOI] [PubMed] [Google Scholar]
- 10.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 11.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197:1701–7. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ancuta P, Wang J, Gabuzda D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J Leukoc Biol. 2006;80:1156–64. doi: 10.1189/jlb.0206125. [DOI] [PubMed] [Google Scholar]
- 13.Weiss JM, Berman JW. Astrocyte expression of monocyte chemoattractant protein-1 is differentially regulated by transforming growth factor beta. J Neuroimmunol. 1998;91:190–7. doi: 10.1016/s0165-5728(98)00183-0. [DOI] [PubMed] [Google Scholar]
- 14.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26:1098–106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randolph GJ, Furie MB. A soluble gradient of endogenous monocyte chemoattractant protein-1 promotes the transendothelial migration of monocytes in vitro. J Immunol. 1995;155:3610–8. [PubMed] [Google Scholar]
- 16.Lin YH, Yang-Yen HF. The osteopontin-CD44 survival signal involves activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. J Biol Chem. 2001;276:46024–30. doi: 10.1074/jbc.M105132200. [DOI] [PubMed] [Google Scholar]
- 17.Katagiri YU, Sleeman J, Fujii H, Herrlich P, Hotta H, Tanaka K, Chikuma S, Yagita H, Okumura K, Murakami M, Saiki I, Chambers AF, Uede T. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999;59:219–26. [PubMed] [Google Scholar]
- 18.Weber C, Belge KU, von Hundelshausen P, Draude G, Steppich B, Mack M, Frankenberger M, Weber KS, Ziegler-Heitbrock HW. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol. 2000;67:699–704. doi: 10.1002/jlb.67.5.699. [DOI] [PubMed] [Google Scholar]
- 19.Braumuller H, Gansauge S, Ramadani M, Gansauge F. CD44v6 cell surface expression is a common feature of macrophages and macrophage-like cells - implication for a natural macrophage extravasation mechanism mimicked by tumor cells. FEBS Lett. 2000;476:240–7. doi: 10.1016/s0014-5793(00)01737-3. [DOI] [PubMed] [Google Scholar]
- 20.Rittling SR, Chen Y, Feng F, Wu Y. Tumor-derived osteopontin is soluble, not matrix associated. J Biol Chem. 2002;277:9175–82. doi: 10.1074/jbc.M109028200. [DOI] [PubMed] [Google Scholar]
- 21.Geissinger E, Weisser C, Fischer P, Schartl M, Wellbrock C. Autocrine stimulation by osteopontin contributes to antiapoptotic signalling of melanocytes in dermal collagen. Cancer Res. 2002;62:4820–8. [PubMed] [Google Scholar]
- 22.Ophascharoensuk V, Giachelli CM, Gordon K, Hughes J, Pichler R, Brown P, Liaw L, Schmidt R, Shankland SJ, Alpers CE, Couser WG, Johnson RJ. Obstructive uropathy in the mouse: role of osteopontin in interstitial fibrosis and apoptosis. Kidney Int. 1999;56:571–80. doi: 10.1046/j.1523-1755.1999.00580.x. [DOI] [PubMed] [Google Scholar]
- 23.Roberts ES, Zandonatti MA, Watry DD, Madden LJ, Henriksen SJ, Taffe MA, Fox HS. Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS. Am J Pathol. 2003;162:2041–57. doi: 10.1016/S0002-9440(10)64336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burdo TH, Marcondes MC, Lanigan CM, Penedo MC, Fox HS. Susceptibility of Chinese rhesus monkeys to SIV infection. Aids. 2005;19:1704–6. doi: 10.1097/01.aids.0000186823.76230.33. [DOI] [PubMed] [Google Scholar]
- 25.Watry D, Lane TE, Streb M, Fox HS. Transfer of neuropathogenic simian immunodeficiency virus with naturally infected microglia. Am J Pathol. 1995;146:914–23. [PMC free article] [PubMed] [Google Scholar]
- 26.Muller WA, Weigl SA. Monocyte-selective transendothelial migration: dissection of the binding and transmigration phases by an in vitro assay. J Exp Med. 1992;176:819–28. doi: 10.1084/jem.176.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–3. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 28.Randolph GJ, Beaulieu S, Pope M, Sugawara I, Hoffman L, Steinman RM, Muller WA. A physiologic function for p-glycoprotein (MDR-1) during the migration of dendritic cells from skin via afferent lymphatic vessels. Proc Natl Acad Sci U S A. 1998;95:6924–9. doi: 10.1073/pnas.95.12.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Randolph GJ, Furie MB. Mononuclear phagocytes egress from an in vitro model of the vascular wall by migrating across endothelium in the basal to apical direction: role of intercellular adhesion molecule 1 and the CD11/CD18 integrins. J Exp Med. 1996;183:451–62. doi: 10.1084/jem.183.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilula NB, Epstein ML, Beers WH. Cell-to-cell communication and ovulation. A study of the cumulus-oocyte complex. J Cell Biol. 1978;78:58–75. doi: 10.1083/jcb.78.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei J, Sun Z, Chen Q, Gu J. Serum deprivation induced apoptosis in macrophage is mediated by autocrine secretion of type I IFNs. Apoptosis. 2006;11:545–54. doi: 10.1007/s10495-006-5146-7. [DOI] [PubMed] [Google Scholar]
- 32.Kiener PA, Davis PM, Rankin BM, Klebanoff SJ, Ledbetter JA, Starling GC, Liles WC. Human monocytic cells contain high levels of intracellular Fas ligand: rapid release following cellular activation. J Immunol. 1997;159:1594–8. [PubMed] [Google Scholar]
- 33.Shoshan Y, Shapira I, Toubi E, Frolkis I, Yaron M, Mevorach D. Accelerated Fas-mediated apoptosis of monocytes and maturing macrophages from patients with systemic lupus erythematosus: relevance to in vitro impairment of interaction with iC3b-opsonized apoptotic cells. J Immunol. 2001;167:5963–9. doi: 10.4049/jimmunol.167.10.5963. [DOI] [PubMed] [Google Scholar]
- 34.Um HD, Orenstein JM, Wahl SM. Fas mediates apoptosis in human monocytes by a reactive oxygen intermediate dependent pathway. J Immunol. 1996;156:3469–77. [PubMed] [Google Scholar]
- 35.Hickey WF. Leukocyte traffic in the central nervous system: the participants and their roles. Semin Immunol. 1999;11:125–37. doi: 10.1006/smim.1999.0168. [DOI] [PubMed] [Google Scholar]
- 36.Bauer J, Wekerle H, Lassmann H. Apoptosis in brain-specific autoimmune disease. Curr Opin Immunol. 1995;7:839–43. doi: 10.1016/0952-7915(95)80057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosel S, Egensperger R, Bise K, Arbogast S, Mehraein P, Graeber MB. Long-lasting perivascular accumulation of major histocompatibility complex class II-positive lipophages in the spinal cord of stroke patients: possible relevance for the immune privilege of the brain. Acta Neuropathol (Berl) 1997;94:532–8. doi: 10.1007/s004010050747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kida S, Steart PV, Zhang ET, Weller RO. Perivascular cells act as scavengers in the cerebral perivascular spaces and remain distinct from pericytes, microglia and macrophages. Acta Neuropathol (Berl) 1993;85:646–52. doi: 10.1007/BF00334675. [DOI] [PubMed] [Google Scholar]
- 39.Wu DT, Woodman SE, Weiss JM, McManus CM, D’Aversa TG, Hesselgesser J, Major EO, Nath A, Berman JW. Mechanisms of leukocyte trafficking into the CNS. J Neurovirol. 2000;6(Suppl 1):S82–5. [PubMed] [Google Scholar]
- 40.Park IW, Wang JF, Groopman JE. HIV-1 Tat promotes monocyte chemoattractant protein-1 secretion followed by transmigration of monocytes. Blood. 2001;97:352–8. doi: 10.1182/blood.v97.2.352. [DOI] [PubMed] [Google Scholar]
- 41.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–4. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 42.Panzer U, Thaiss F, Zahner G, Barth P, Reszka M, Reinking RR, Wolf G, Helmchen U, Stahl RA. Monocyte chemoattractant protein-1 and osteopontin differentially regulate monocytes recruitment in experimental glomerulonephritis. Kidney Int. 2001;59:1762–9. doi: 10.1046/j.1523-1755.2001.0590051762.x. [DOI] [PubMed] [Google Scholar]
- 43.Giachelli CM, Lombardi D, Johnson RJ, Murry CE, Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am J Pathol. 1998;152:353–8. [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu B, Suzuki K, Goldberg HA, Rittling SR, Denhardt DT, McCulloch CA, Sodek J. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. J Cell Physiol. 2004;198:155–67. doi: 10.1002/jcp.10394. [DOI] [PubMed] [Google Scholar]
- 45.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 46.Scatena M, Almeida M, Chaisson ML, Fausto N, Nicosia RF, Giachelli CM. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141:1083–93. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]