Abstract
Immune dysregulation initiated by a profound loss of CD4+ T-cells is fundamental to HIV-induced pathogenesis. Infection of domestic cats with a non-pathogenic lentivirus prevalent in the puma (puma lentivirus, PLV or FIVPCO) prevented peripheral blood CD4+ T-cell depletion caused by subsequent virulent FIV infection. Maintenance of this critical population was not associated with a significant decrease in FIV viremia, lending support to the hypothesis that direct viral cytopathic effect is not the primary cause of immunodeficiency. Although this approach was analogous to immunization with a modified live vaccine, correlates of immunity such as a serum-neutralizing antibody or virus-specific T-cell proliferative response were not found in protected animals. Differences in cytokine transcription profile, most notably in interferon gamma, were observed between the protected and unprotected groups. These data provide support for the importance of non-adaptive enhancement of the immune response in the prevention of CD4+ T-cell loss.
Keywords: FIV, CD4+ T-cell, cross-species lentivirus infection, interferon, innate immunity
Introduction
Dysregulation of the immune response brought on by the loss of CD4+ T-cells is the initiator of progression to Acquired Immunodeficiency Syndrome (AIDS) following infection with human immunodeficiency virus (HIV) (Alimonti, et al. 2003; Douek, et al. 2003; Lackner and Veazy, 2007), virulent simian immunodeficiency virus (SIV) infection in Asian macaques (Lackner and Veazy, 2007; Viollet, et al., 2006) and virulent feline immunodeficiency virus (FIV) infection in domestic cats (Bendinelli, et al., 1995; Egberink and Horzinek, 1992). Prevention of CD4+ T-cell loss following HIV infection has not been achieved despite more than 20-years of vaccine research, although multi-drug therapy has proven successful in extending patient survival (Lima, et al., 2007; Rogers, et al., 2000). Reduction in viral load or preservation of CD4+ T-cells has been noted in a small percentage of animals in individual vaccine studies using SIV or simian/human virus hybrids in non-human primate models (Ahmed et al., 2002; Connor, et al., 1998; Stolte-Leeb, et al., 2006). Due in part to this variation in response these models have not yet revealed an obvious approach or an immune response to target in order to prevent infection or the development of AIDS (Davenport, et al., 2007; Douek, et al., 2003; Lackner and Veazy, 2007; McMichael, 2006) . Further, the cause of the CD4+ T-cell depletion during HIV infection is controversial (Alimonti, et al., 2003; Hel et al., 2006). Viral infection can directly cause the death of these cells (Alimonti, et al., 2003); however following recognition that the rate of cell death far exceeds the infection rate, it has been postulated that the chronic nature of the immune response and induction of apoptosis in activated bystander cells are at least in part responsible for the disease state resulting from virulent HIV and SIV infections (Anderson, et al., 1998; Douek, et al., 2003; Hel, et al., 2006), recently it has been recognized that even the chronic state of activation may not explain the kinetics of CD4+ T-Cell depletion (Yates, et al., 2007).
FIV infection has many analogies to HIV infection; the virus has emerged relatively recently, targets similar cell types, and the disease state demonstrates a similar time course, clinical signs and outcome (Bendinelli, et al. 1995; Overbaugh, et al., 1997; Siebelink,et al.,1990). Concurrently, FIVs which are endemic to the non-domestic cat population parallel SIVagm and other naturally occurring SIVs in that they are minimally pathogenic in their natural host (VandeWoude and Apetrei, 2006). Our previous work indicates that infection of domestic cats with a lentivirus native to the puma (FIVPCO Strain PLV-1695, subsequently referred to as PLV) results in mild lymphadenopathy and only a slight, transient decrease in CD4+ T-cell count. PBMC viral burden gradually decreases over the course of several months of infection and is ultimately virtually eliminated by the host without evidence of strong adaptive immune activation. In late infection, proviral burden persisted in gastro-intestinal vs lymphoid compartments (TerWee, et al., 2005). In this study, domestic cats were inoculated with PLV or sham inoculum (n=10/group). After infection was established, five animals from each group were inoculated with virulent FIV and monitored for clinical signs, peripheral blood cell counts, CD4+ and CD8+ T-Cell counts, cellular and plasma viral loads, humoral and cellular immune response, and PBMC cytokine expression. A highly pathogenic molecular clone of FIV subtype C which induces high viral loads and significant CD4+ T-Cell loss early after infection (De Rozières, et al., 2004) was used as the challenge virus. Protection against CD4+ T-cell depletion was afforded by prior infection with a non-pathogenic lentivirus using the domestic cat model of immunodeficiency virus pathogenesis.
Results
PLV infection was uniformly detected by 3 days post inoculation
Figure 1 demonstrates mean viral copy number in peripheral blood mononuclear cells (PBMC) from cats inoculated with PLV on day 0 as assessed by real time PCR specific for PLV-1695. Provirus was detected in all PLV-inoculated cats by 3 days after inoculation, while virus was not detected in sham-inoculated controls or animals only infected with FIV at any time point. Copy number peaked at an average of 1 copy per 500 cells at day 10 and then declined, becoming no longer regularly detectable in some individual animals beyond day 37. Cellular virus was still detectable in 4 animals at levels of 1 copy per 1000 cells to 1 copy per 100,000 cells at the last time point (day 108).
Figure 1.
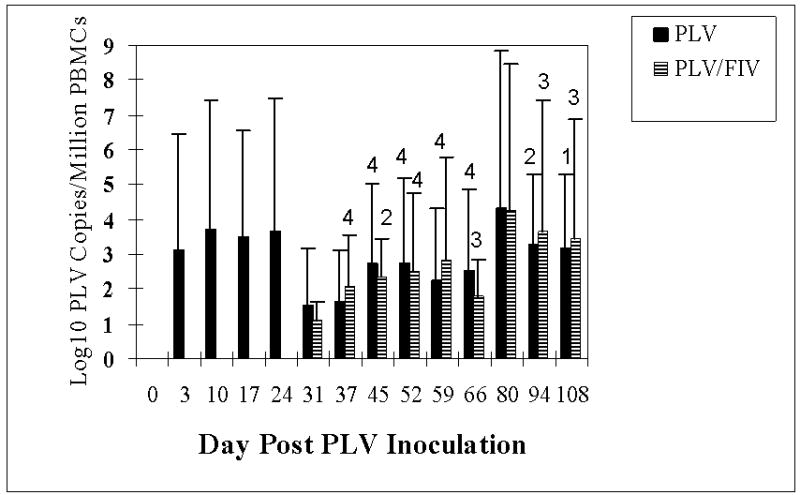
Mean PLV viral copy number per 1 million PBMCs as measured by real-time PCR in groups inoculated with PLV or medium only on day 0. Bars indicate the standard deviation. Arrow indicates the inoculation of 5 animals with FIV (striped bars). N = 10 for days 3, 10, 17 and 24 and n = 5 for day 31 and thereafter. A number less than 5 above the standard deviation bar indicates the number of animals that had copy numbers above the lower limit of detection. Calculation of means used values for only those animals with detectable virus.
Prior PLV infection rescued CD4+ T-cell depletion following virulent FIV infection
Complete white blood cell counts with differentials were conducted to determine if the kinetics of CD4+ T-Cell depletion in FIV-C infection was altered by prior PLV infection. As shown in Figure 2 and consistent with previous observations, CD4+ T-cell count was not significantly decreased by infection with PLV (study days 10, 17 and 24) and in fact there was a significant increase in CD4+ T-cell count in PLV-only inoculated animals at day 31. Lymphocyte and white blood cell (data not shown) counts were also unchanged following PLV inoculation. One-half of the PLV-infected animals and one-half of the sham-inoculated controls were inoculated with FIV on day 28 (indicated by an arrow). The PLV-negative, FIV-inoculated cats (solid triangle) demonstrated a marked decline in CD4+ T-cell count by 3 days after FIV inoculation which persisted for the course of the study. The differences attained statistical significance on days 3, 9, 17, 31, 38 and 66 post FIV (study days 31, 37, 45, 59, 66 and 94). This decline in CD4+ T-cell count was reflected in total lymphocyte count with a statistically significant decrease on days 31, 37, 45, 52 and 59 (data not shown). In contrast, PLV-infected cats challenged with FIV(solid gray circles) showed a statistically significant decline in CD4+ T-cell count only at day 37 (9 days post FIV-inoculation) and a decrease in lymphocyte count on days 37 and 45. Control animals also demonstrated a statistically significant increase in CD4+ T-cell count on study day 52. White blood cell count (data not shown) was significantly higher in PLV-only infected animals on study days 31 and 52, significantly lower in FIV-infected animals on study days 37, 59 and 66 and significantly lower in dual-infected animals on study days 59, 66 and 94. In summary, PLV infection did not result in CD4+ T-Cell depletion, and prevented the sustained loss brought about by FIV-C infection in all animals.
Figure 2.
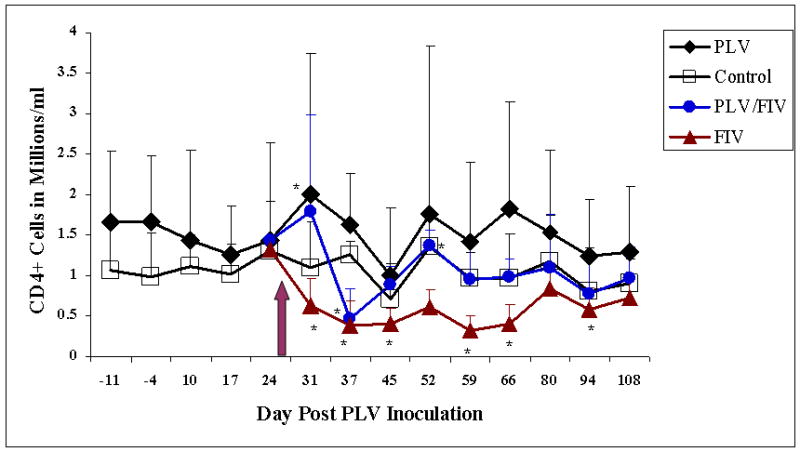
Mean CD4-positive T-Cell counts in groups inoculated with PLV or medium only on day 0. Solid diamonds represent the PLV-inoculated group and open boxes represent the medium-inoculated controls. Arrow indicates inoculation of 5 animals from each group with FIV or diluent. Solid triangles represent the single virus inoculated group (FIV only) and shaded circles represent the dual virus inoculated group. Asterisks indicate a statistically significant difference (p≤0.05) from baseline values.
FIV PBMC proviral load is slightly diminished in cats previously inoculated with PLV
FIV-C in PBMCs and in plasma was measured by real-time PCR to determine if prior PLV infection inhibited FIV infection. FIV was not detected in either group at day 31 (3 days after FIV inoculation). However, as shown in figure 3A, both PLV and sham-inoculated controls had a similar FIV copy number in PBMCs on day 37 (9 days after inoculation). FIV viral copy number in PBMCs was statistically significantly lower in PLV-inoculated animals only on study day 52. However there was a trend for FIV copy number to be lower in PLV-inoculated animals throughout the study, with p values of 0.071 and 0.100 on study days 45 and 66 respectively. FIV plasma viremia was measured on study days 37, 59 and 80. As shown in Figure 3B, these results support the lack of overall reduction in FIV replication by previous PLV infection as there were no significant differences between the two groups at the time points examined. Sham inoculated animals, and those only infected with PLV, were FIV negative at all time points when evaluated by real time PCR (data not shown).
Figure 3.
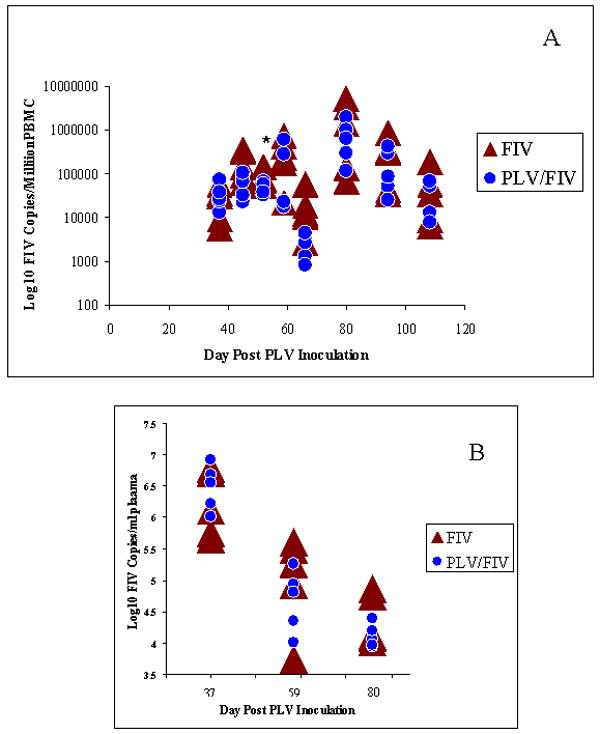
FIV Infection. FIV copy number per 1 million PBMCs (A) and FIV copy number per milliliter of plasma (B) as measured by PCR in individual animals inoculated with PLV on day 0 followed by FIV on day 28 (shaded circles) or medium only on day 0 followed by FIV on day 28 (solid triangles). Asterisk indicate the single time point at which the higher copy number in the FIV only group were statistically significantly higher than in the group inoculated with PLV prior to FIV.
Protection from CD4 depletion does not correlate with acquired immunity
To determine if prior infection with PLV primed an antibody or T-Cell response to FIV, we examined SN antibody 3 weeks after FIV inoculation. As shown in figure 4, FIV SN antibody titer at day 59 (day 31 post FIV) was not significantly different in cats inoculated with FIV compared to those infected with PLV prior to FIV, indicating no anamnestic response to FIV following PLV infection. FIV SN titer ranged from 1:5 to 1:70 in PLV infected animals and 1:3 to 1:20 in FIV infected animals. Only 1 PLV single-virus-inoculated animal had a detectable SN response to FIV.
Figure 4.
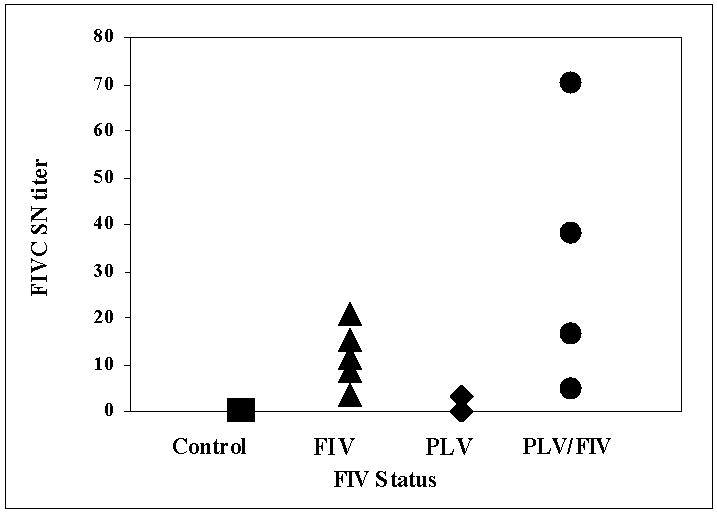
Serum Neutralizing Antibody (SN) titer measured 59 days post PLV inoculation in individual animals inoculated with PLV on day 0 followed by FIV on day 28 (circles), medium only on day 0 followed by FIV on day 28 (triangles), PLV on day 0 followed by 0.9% NaCl on day 28 (diamonds) or medium only on day 0 followed by 0.9% NaCl on day 28 (squares). There was no statistically significant difference between groups.
Lymphocyte proliferative response to mitogen or antigen was determined at various study time points (Fig. 5). Although the group infected with FIV had the lowest Concanavalin A (Con A) response at most time points, there were no statistical differences as compared to controls in response in any group in proliferation to Con A (study days -4, 17, 31, 45 and 66). The group inoculated with FIV following PLV infection had a significantly higher Con A response as compared to controls on day 59 (Fig. 5A). PLV-inoculated animals had a higher proliferative response to both FIV and PLV on day 17, but this difference did not achieve statistical significance. Similarly, although animals inoculated with FIV had a high proliferative response to both FIV and PLV on study day 59, this difference did not achieve statistical significance.
Figure 5.
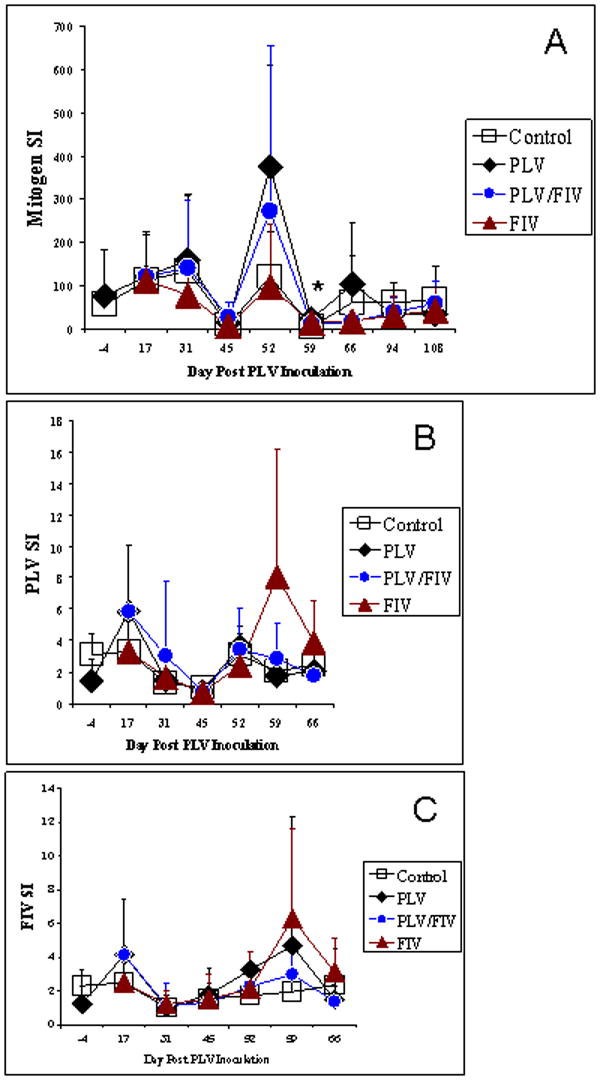
Lymphocyte blastogenesis response to mitogen or antigen as measured by incorporation of 3H thymidine. PBMCs were stimulated in triplicate with 1 μg of inactivated PLV or FIV for 4 days or with 1.5 μg Con A for 3 or 4 days prior to incubation in the presence of 3H thymidine and harvest. Stimulation indices (SI) were calculated by dividing the average count for each agent by that of the average count for unstimulated cells. Group mean SI are shown for A) mitogen B) PLV or C) FIV in groups inoculated with PLV or medium only on day 0. Solid diamonds represent the PLV-inoculated group and open boxes represent the medium-inoculated controls. Arrow indicates inoculation of 5 animals from each group with FIV or diluent. Solid triangles represent the single virus inoculated group (FIV only) and shaded circles represent the dual virus inoculated (PLV followed by FIV) group. As indicated by an asterisk, the only statistically significant difference was in Con A response on Day 59 in the PLV only group (SI of 23) was compared to the control group (SI of 5).
CD4+ T-cell maintenance is associated with Interferon gamma (IFN-γ) expression
To determine if protection was associated with a strong TH-1 or TH-2 cytokine response, cytokine mRNA (IL-10, IL-4, IL-12, TNF-α, and IFN-γ) was measured using real time PCR from bulk PBMC. In order to normalize for differences in measured cytokine expression between sample days, cytokine levels are expressed as fold increase over values at the same time point for controls. As shown in Figure 6, cats inoculated with PLV had a statistically significant fold increase from controls in IFN-γ expression on study days 10, 17, 31, 37, 52 and 59. FIV inoculation was also followed by an increase in IFN-γ expression, but not until day 52, (24 days after FIV inoculation). 9/10 of PLV-inoculated animals exhibited a 2-fold or greater (range = 0.8-10.8, mean = 4) increase in IFN-γ between days 3 and 31. PLV-inoculated animals thus had significantly higher IFN-γ levels not only at the time of FIV exposure, but for over 3 weeks following FIV inoculation. Though samples were not available from all animals for analysis at day 108, the trend was for IFN-γ mRNA levels to return to baseline for all infected groups (data not shown).
Figure 6.
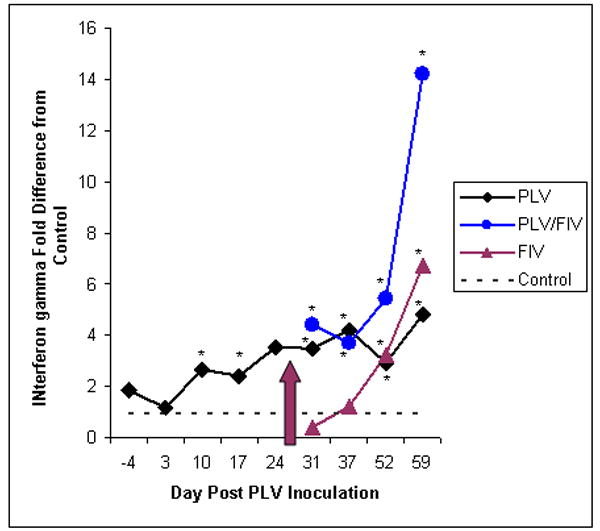
IFN-γ expression was measured by real time PCR. Level of expression relative to that of the control group is shown as group means for groups inoculated with PLV or medium only on day 0. Solid diamonds represent the PLV-inoculated group. Arrow indicates inoculation of 5 animals from each group with FIV or diluent. Solid triangles represent the single virus inoculated group (FIV only) and shaded circles represent the dual virus inoculated (PLV followed by FIV) group. Asterisks indicate statistically significant differences (p ≤0.05) from the control group. The number of samples tested for each group at each time point was 10 (before day 28) or 5 (after day 28) with the following exceptions: day 3 n = 8 for control, day 10 n = 9 for control, day 17 n = 8 for control, day 31 n = 3 for control and n = 4 for FIV.
IL-10 (data not shown) was significantly elevated on study day 52 in the FIV-inoculated animals. IL-4 expression (data not shown) was slightly elevated in the groups receiving PLV from day 10 onward, achieving statistical significance on day 31. IL-4 was more variable in the FIV-only group, falling below the control levels on day 52 and above on days 31 and 59, but not significantly so. IL-12 expression (data not shown) was not statistically different from control levels in any group at any time point. TNF expression was also variable demonstrating a statistically significant difference only prior to and day 10 after PLV inoculation.
PLV infection did not prevent neutropenia subsequent to infection with FIV
Neutrophil counts declined precipitously in FIV-inoculated groups with or without prior PLV inoculation (Fig. 7). Neutropenia was significant relative to baseline in PLV-infected, FIV –inoculated animals on days 59 and 66 and in FIV-infected animals on days 66 and 80. The diminished neutrophil count in these groups coincides with a statistically significant decrease in WBC count on days 59 and 66 for the PLV/FIV group and on day 66 for the FIV-only group (data not shown). The control group demonstrated a significant neutrophilia on day 45 while PLV –only animals experienced neutrophilia relative to baseline on post-inoculation days 37 and 45.
Figure 7.
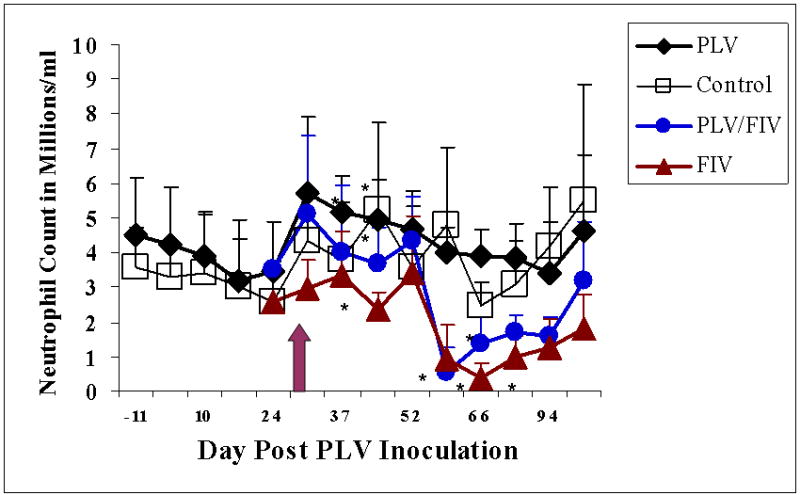
Mean neutrophil counts in groups inoculated with PLV or medium only on day 0. Solid diamonds represent the PLV-inoculated group and open boxes represent the medium-inoculated controls. Arrow indicates inoculation of 5 animals from each group with FIV or diluent. Solid triangles represent the single virus inoculated group (FIV only) and shaded circles represent the dual virus inoculated (PLV followed by FIV) group. Asterisks indicate a statistically significant difference (p≤0.05) from baseline value.
Clinical signs were unremarkable
Mild lymphadenopathy was noted following PLV inoculation. FIV inoculation resulted in mild to moderate lymphadenopathy in both PLV- inoculated and sham-inoculated animals.
Discussion
Previous infection with a non-pathogenic lentivirus protected against the significant and prolonged peripheral blood CD4+ T-cell depletion that occurs following inoculation with virulent FIV, consistent with results of previous studies (VandeWoude, et al., 2002). Preservation of CD4+ lymphocytes did not correspond to an immediate or sustained significant decrease in peripheral viral load as measured by viremia or intracellular (PBMC) virus and was not dependent upon a high PBMC PLV viral load at the time of FIV inoculation.
This disconnect between pathogenic viral load and CD4+ T-cell depletion is consistent with results for the natural course of HIV infection, in which it has been shown that CD4+ T-cell decline can not be predicted by viral load in untreated HIV infection (Rodŕiguez, et al., 2006); further, although infection with viral strains such as subtype D results in a faster rate of CD4+ T-cell decline and more rapid progression of disease, set-point as well as early viral loads are equivalent to those in infection with less pathogenic strains (Baeten, et al., 2007). Similarly, in non-pathogenic SIV infection, consistently high viral loads are not associated with peripheral blood CD4+ T-cell loss or with the development of AIDS (Broussard, et al., 2001; Chakrabarti, 2004; Rey-Cuille, et al., 1998). Additionally, HIV- infected patients undergoing treatment with anti-retroviral agents typically experience a reduction of viral load to the limit of detection without complete recovery of CD4+ T-cells (Goicoechea, et al., 2006). Data from other studies suggest a correlation between viral load and CD4+ T-cell loss (Goldstein, et al, 2005; Mellors, et al., 1997), and undeniably viral infection initiates the destruction of this cell population. It is therefore evident that there are factors yet to be discovered to explain the complex association of viral load with CD4+ T-Cell loss.
As might be predicted from our previous observations that PLV does not induce a vigorous immune response during domestic cat infection (TerWee, et al., 2005), and that the homology between FIV and PLV env is low (Smirnova, et al., 2005), prevention of CD4 + cell loss in this feline model was not linked to a cross-neutralizing immune response to FIV in PLV-infected cats. An anamnestic virus neutralizing antibody response was not observed after FIV inoculation in the animals infected with PLV, and enhanced lymphocyte proliferation to virus specific antigen was not detected. Although additional measures may reveal a role for adaptive immunity in the mechanism of protection against lentivirus-induced immunodeficiency, our results are consistent with those reported in many HIV, SIV and FIV studies that fail to show a clear cause-and-effect relation between protection and a robust immune response (Amara, et al., 2005; Hosie et al., 1998; Langlois, et al., 1998; Singh, et al., 2005; Stebbings, et al., 2002). In a recent study, non-progressors and patients treated with HAART actually had lower neutralizing antibody responses to autologous virus than untreated viremic patients, indicating that the viral replication was driving the antibody response rather than being controlled by it (Bailey, et al., 2006). This has also been documented in SIV: An antibody response was detected after challenge exposure in non-vaccinated groups and in vaccinated progressors, but not in the vaccinated and protected non-progressors (Kawada, et al., 2007). Others however have noted a correlation between induction of a post-vaccinal neutralizing antibody response against the challenge strain and reduction in viral replication (Quinnan, et al., 2005), a correlation between antibody avidity and rate of disease progression (Korthels, et al., 2006), and greater loss of CD4+ T-cells and increased viral replication after experimental B-cell depletion (Miller, et al., 2007), indicating that an antibody response may be effective if it reaches a certain threshold of quality and quantity. In light of the envelope diversity of circulating strains of HIV, the goal of many vaccine research programs is a cytotoxic T-cell response (Davenport, et al., 2007; McMichael, 2006). Disparate results for the effectiveness of this type of immune response are also evident. Although depletion of CD8 + T-cells following SIV infection has been shown to cause an increase in virus replication (Schmitz, et al., 2005), vaccines which induce high levels of CD8+ T-cells have not been able to prevent infection or disease and CD4+ T-cell loss (McMichael, 2006). Even though there is an overall reduction in chronic viral replication, CTL escape mutants emerge (Loffredo, et al., 2007; Mandl, et al., 2007) and an effective response is achieved too late (Davenport, et al., 2007).
In contrast to the lack of correlation between adaptive immune response induction by PLV and down-regulation of PLV or protection against FIV challenge, we have documented changes in recombination rates, genomic selection, and mutation rate in PLV genomes following passage through domestic cats, notable at key residues in pol and signified by G to A conversion (Poss, et al., 2006, Poss, et al, 2007). This accumulated evidence strongly supports innate and intracellular anti-retroviral defenses are enhanced in the face of PLV infection in domestic cats—supporting these host defenses as primary mechanisms preventing pathogen infection of non-target species.
Prevention of superinfection is documented as a means by which lentiviruses increase fidelity. HIV downregulates the expression of its binding receptor, CD4, during its replicative cycle (Lama, 2003; Lindwasser, et al., 2007). Although we have observed reduced FIV replication after PLV inoculation in vitro (VandeWoude, et al., 2002), PLV and FIV appear to use different receptors (Smirnova, et al., 2005). Additionally, since the number of PLV-infected cells was low at the time of FIV inoculation a global antiviral state induced by PLV infection seems a more likely explanation for our observations than a direct block to superinfection. In this context the ‘non-adapted’ pathogen is prevented from replication and/or successful infection because the new host has molecular machinery able to interfere with the lentiviral lifecycle. It is interesting to speculate that these non-specific innate factors, activated during PLV infection because of host:pathogen discordance, modify the host environment to partially cripple subsequent virulent FIV infection. While we did not see an overall significant reduction in FIV viral load in circulation, it is possible that the viruses being produced were less fit due to residual intracellular restriction mechanisms induced by PLV infection. Evaluation of FIV genomes for evidence of hypermutation and recombination will determine this possibility.
It is also plausible that replication of virulent FIV was abrogated at key sites of early replication other than PBMCs. Although high levels of FIV are found in bone marrow (Beebe, et al., 1992; Dua et al., 1994; S. Troth, unpublished data), we have previously shown that PLV can not be detected in samples of bone marrow harvested 6 months after PLV-inoculation; however, proviral is maintained in gastrointestinal tract and associated lymph nodes (TerWee, et al., 2005). Lack of co-localization of the two viruses in bone marrow may explain the absence of protection from neutropenia in dual-virus infected animals; further, it is possible that FIV replication in FIV-PLV dual infected cats is limited in sites such as the gastrointestinal tract due to primary interference with PLV, or redistribution of FIV target cells, preserving CD4+T cell populations that ultimately circulate peripherally. Quantitation of both PLV and FIV in lymphoid and gastrointestinal tissues during single and dual infection will provide informative clues about mechanisms underlying CD4+ T-Cell loss in FIV infected animals.
IFN-γ expression was increased in PBMC by both pathogenic and non-pathogenic virus exposure, however animals protected from CD4+ T-cell lymphopenia had a higher level of IFN-γ mRNA prior to exposure to virulent virus. The role of IFN-γ in protection from feline or human AIDS is not clear. Vaccination with a DNA vaccine expressing IFN-γ has been shown not to reduce viremia following exposure to FIV (Gupta, et al., 2007). However, effect on CD4+ T-Cell count was not determined since this challenge virus did not induce measurable CD4 +T-cell loss. In HIV infected individuals, CD4+ T-cell decline was not found to correlate with HIV-specific IFN-γ secretion (Peretz, et al., 2005) and interferon gamma is at higher levels in pathogenic cross-species SIV infection than in non-pathogenic infection, indicating that an activated immune system is prognostic of pathogenesis (Kornfeld, et al., 2005). Conversely, other studies support our results of an association between IFN-γ and diminished pathogenesis. Vaccine-induced antigen-specific IFN-γ secretion has been shown to correlate with survival, protection and reduced viral loads following challenge with SIV or SHIV (Abel, et al., 2003; Boyer, et al., 2006; Sun, et al., 2006). Given that others have observed induction of cytidine deaminase by treatment with IFN-γ in vitro (Jost, et al., 2007) it is possible that the protection we observed in conjunction with increased IFN-γ is related to synergy between innate pathways of altered cytokine expression and intracellular restriction in individuals exposed to certain pathogens.
The feline model provides a well controlled system to examine pathogenesis, protection and early effects of infection with viruses which induce immunodeficiency. Prior infection with a minimally pathogenic virus from a related species provided solid protection from CD4+ T-cell loss. Our data lend support to the body of evidence that decouples viral replication and peripheral blood CD4+ T-cell loss. Our data also support the lack of either an antibody or cell mediated immune response in the responsibility for maintenance of this population of cells. Further studies dissecting potential mechanisms for our observations will provide fundamental knowledge toward the understanding of the pathogenesis of AIDS.
Materials and methods
Viral Stocks
Table 1 describes the viral stocks used in this study. The MYA-1 cell line is of domestic cat PBMC origin (Miyazawa, et al., 1989). PLV was isolated from a co-culture of puma and domestic cat PBMCs and was expanded in MYA-1 cells. FIV-C was recovered from the retropharyngeal lymph node of a cat inoculated with a molecularly cloned FIV-C by culture with MYA-1 cells. Reverse transcriptase (RT) activity of culture supernatants was monitored and virus was harvested by slow speed (200-500xg) centrifugation using a Beckman GPR centrifuge with a GH 37 rotor (Beckman Coulter, Fullerton, CA) to remove cell debris at peak RT activity. The sham inoculum (medium) was similarly prepared from culture supernatant of un-infected MYA-1 Cells.
Table 1.
Description of Virus Stocks
| Stock | TCID50/ml1 | Days in culture2 | Copies/ml3 | RT activity4 | Passage history | In vivo infectivity |
|---|---|---|---|---|---|---|
| PLV-1695 | 104.7 | 12 | 5 × 106 | 5847 | Puma PBMC co-cultivated with domestic cat cells | 1 ml infected 14/14cats IV and 4/4 ON |
| FIV-C | 107.2 | 17 | Not done | 19628 | Retropharyngeal lymph node from cat noculated with molecular clone cultured with domestic cat cells | 1 ml of 1:100 dilution nfected 11/11 cats IV and 2/2 ON |
Titer calculated on MYA-1 cells.
Days in culture for final passage.
Calculated by real-time PCR.
Calculated by micro-titer reverse transcriptase assay.
Animals
20 specific-pathogen-free (SPF) cats were obtained from a breeding colony at Colorado State University. Animals were randomized by litter and gender and were housed in groups of 5 in pens in isolation rooms in an AAALAC-international accredited animal facility. All procedures were approved by the CSU Institutional Animal Care and Use Committee prior to initiation.
Study Design
Blood samples were obtained by venipuncture of the jugular or cephalic vein on study days -11, -4, 3, 10, 17, 24, 31, 37, 45, 52, 59, 66, 80, 94, and 108 (relative to PLV exposure). On Day 0, ten 14-22 week-old cats were inoculated IV with 1 ml of PLV while the remaining 10 cats received culture supernatant from un-infected MYA-1 cells IV.Twenty-eight days later, 5 of the PLV-inoculated animals and 5 of the sham-inoculated controls received 1 ml of FIV stock which had been diluted 1:100 in 0.9% NaCL solution IV and the remaining animals were inoculated by the same route with 0.9% saline. Thus study termination was 108 days post PLV inoculation and 80 days post FIV challenge.
Clinical signs
Animals were observed for clinical signs at least daily throughout the study. Lymph nodes were palpated at least weekly. Body weights were measured weekly from 4 weeks prior to until 12 weeks post PLV exposure.
Viral Copy Number
A real time PCR standard curve generated from serial dilutions of feline PBMC from 1000 to 5 × 106 subjected to real time PCR for the cellular house-keeping gene, Glyceraldehyde-3-Phosphate Dehydrogenase (GADPH) as described by Leutenegger et al. (1999). Peripheral blood mononuclear cells (PBMC) from study animals were purified from heparinized whole blood samples from experimental animals using a Histopaque (Sigma, St. Louis, MO) gradient according to the product insert. DNA was extracted from 1 million PBMCs using the Qiamp blood mini DNA kit (Qiagen, Valencia, CA). DNA from each sample was eluted with 50-200 μl of buffer and PLV in 5 μl of sample was quantitated in triplicate using real time PCR with plasmid encoded PLV as a positive control. Primers and probe were designed for the pol region of PLV 1695. This assay has a less than 3% within and between sample variance and is sensitive to a minimum of 10 copies. The difference in amplification efficiency for plasmid vs sample DNA was less than 1% (Sondgeroth, et al., 2005). Primers and probe for FIVC gag (Pedersen, et al., 2001) were used to quantitate FIV in PBMCs and plasma. The sensitivity of detection is a minimum of 5 copies. Due to substantial heterogeneity between FIVC and PLV 1695, primers and probes are specific for each virus and do not cross-amplify (reported in results). DNA samples were also subjected to GAPDH real time analysis and values normalized to GAPDH standard curve to determine the number of cell equivalents per DNA sample; proviral copy number per cell was calculated on this basis.
To determine the number of viral copies in plasma, RNA in 140 μl of EDTA anticoagulated plasma was purified using the Qiamp Viral RNA Mini Kit (Qiagen, Valencia, CA). Following treatment with DNase (DNA-free, Ambion, Austin, TX), RNA was transcribed to DNA using Superscript II (Invitrogen) and then was quantitated using real time PCR The standard curve was generated using dilutions of a viral stock which was quantitiated against a DNA plasmid standard curve, diluted in negative plasma and then extracted using the same procedure as samples. All samples collected on a given study day were prepared and tested together.
Cytokines
Cytokines were quantitated by real time PCR using the method of Leutenegger et al. (1999). Five to ten million PBMCs (purified as above) were dissolved in Trizol (Sigma, St. Louis, MO) at 10 million cells/ml. cDNA was reverse transcribed from RNA purified by extracted phenol:chloroform extraction and ethanol precipitation. Cytokine expression for IL-10, IL12p40 and Interferon gamma (IFN-γ) was quantitated relative to that of the gene, GAPDH using the formula 2-ΔCT, where ΔCT represents the cycle at which threshold is reached for the GAPDH is subtracted from the cycle at which threshold is reached for the cytokine. All samples collected on a given study day were prepared and tested together.
Hematology
Total white and red blood cell counts were measured using a Coulter Z1 (Coulter, Miami, FL). Differential counts were performed manually and the percentage s of lymphocytes positive for CD4, CD8 and CD25 were determined by flow cytometry using monoclonal antibodies to feline CD4 and CD8 (Southern Biotech, Birmingham, AL). Two to five × 105 PBMCs were incubated for 20-60 minutes at room temperature in monoclonal antibody at 5 μg/ml in flow buffer (PBS containing 2% FBS and 0.2% sodium azide). Cells were then washed twice in flow buffer, resuspended in 100 μl of fluorescein-labeled sheep anti-mouse IgG (Sigma, St. Louis, MO) at 10 μg/ml in flow buffer and incubated for 20-60 minutes at room temperature in the dark. Cells were washed once in flow buffer and then analyzed with a Coulter EPICS XL MCL flow cytometer (Beckman Coulter, Miami, FL). List mode files were analyzed using FlowJo (Tree Star Inc., San Carlos, CA). Total cell counts for each phenotype were calculated by multiplying the total white blood cell count by the percentage of lymphocytes in the sample as determined by the differential count and then by the percentage of lymphocytes expressing that phenotype.
Virus-neutralizing antibody (VN)
VN titer was determined by adding a constant amount of virus to serial two fold dilutions of plasma, starting at a 1:5 final dilution. Following a 90-minute incubation at 37°C with 5% CO2, 100 μl of the virus/plasma mixture was added in triplicate to MYA-1 cells seeded at 1 × 105 cells/well in 96 well plates. The virus inoculum was titrated in triplicate using 10 fold serial dilutions. Virus was detected by reverse transcriptase assay after 14 days incubation at 37 °C with 5% CO2 and titers of virus inoculum and antibody were calculated using the Spearman-Kärber method (Blake and O’Connell, 1993).
Lymphocyte blastogenesis assay
0.1 million PBMCs were incubated in triplicate with medium alone, 1μg of PLV or FIV inactivated using AT-2 (Rossio et al., 1998) and 1.5 μg of Concanavalin A (Con A). 3H at 1μCi/well was then added 3 (Con A days -4, 17, 37, 45 and 52, 94 and 108) or 4 (antigen, PMA, and days 59 and 66 for Con A) days later and the cells were harvested onto filter paper using a 96 well cell harvester (Wallac, Turku, Finland) 20 hours later. Stimulation indices were calculated by dividing the count for each sample by the count with medium alone.
Statistical Analysis
Repeated-measures analysis of variance (ANOVA) was used to determine if significant differences (p< 0.05) could be attributed to treatment or time. If a significant difference was detected, individual comparisons of groups or time points were conducted using the Student t test or Student t test for paired data as appropriate. All analyses were performed using Microsoft Excel (Microsoft Corporation, Redmond WA.)
Acknowledgments
This work was supported by grant R01-AI-52055 from DIADS, NIAID, NIH. The authors express their gratitude to Jeanette Hayes-Klug and staff for attentive animal care and to the laboratories of Drs. E.A. Hoover and P. R. Avery, especially Kevin O’Halloran for FIV-C specific primer and probe and Candace Mathiason for tissue used to amplify the FIV-C viral stock.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel K, Compton L, Rourke T, Montefiori D, Lu D, Rothaeusler K, Fritts L, Bost K, Miller C. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J Virol. 2003;77:3009–3118. doi: 10.1128/JVI.77.5.3099-3118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed RK, Makitalo B, Karlen K, Nilsson C, Biberfeld G, Thorstensson R. Spontaneous production of RANTES and antigen-specific IFN-gamma production in macaques vaccinated with SHIV-4 correlates with protection against SIVsm challenge. Clin Exp Immunol. 2002;129:11–18. doi: 10.1046/j.1365-2249.2002.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimonti JB, Ball TB, Fowke KB. Mechanisms of CD4+ T Lymphocyte cell death in human immunodeficiency virus infection and AIDS. J Gen Virol. 2003;84:1649–1661. doi: 10.1099/vir.0.19110-0. [DOI] [PubMed] [Google Scholar]
- Amara RR, Patel K, Niedziela G, Nigam P, Sharma S, Staprans SI, Montefiori DC, Chenareddi L, Herndon JG, Robinson HL, McClure HM, Novembre FJ. A combination DNA and attenuated simian immunodeficiency virus vaccine strategy provides inhanced protection from Simian/Human Immunodeficiency virus-induced disease. J Virol. 2005;79:l5356–15367. doi: 10.1128/JVI.79.24.15356-15367.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RW, Ascher MS, Sheppard HW. Direct HIV cytopathicity cannot account for CD4 decline in AIDS in the presence of homeostasis: a worst-case dynamic analysis. JAIDS. 1998;17:245–252. doi: 10.1097/00042560-199803010-00010. [DOI] [PubMed] [Google Scholar]
- Bailey JR, Lassen KG, Yang H-C, Quinn TC, Ray SC, Blankson JN, Siliciano RF. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active retroviral therapy. J Virol. 2006;80:4758–4770. doi: 10.1128/JVI.80.10.4758-4770.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten JM, Chohan B, Lavreys L, Chohan V, McClelland RS, Certain L, Mandaliya K, Jaoko W, Overbaugh J. HIV-1 Subtype D infection is associated with faster disease progression than subtype A in spite of similar plasma HIV-1 loads. JID. 2007;195:1177–1180. doi: 10.1086/512682. [DOI] [PubMed] [Google Scholar]
- Beebe AM, Gluckstern TG, George J, Pedersen NC, Dandekar S. Detection of feline immunodeficiency virus infection in bone marrow of cats. Vet Immunol Immunopathol. 1992;35:37–49. doi: 10.1016/0165-2427(92)90119-b. [DOI] [PubMed] [Google Scholar]
- Bendinelli M, Pistello M, Lombardi S, Poli A, Garzelli C, Matteucci D, Ceccherini-Nelli L, Malvaldi G, Tozzini F. Feline immunodeficiency virus: an interesting model for AIDS studies and an important cat pathogen. Clin Microbiol Rev. 1995;8:87–112. doi: 10.1128/cmr.8.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer FD, Robinson TM, Maciag PC, Lewis MG, Weiner DB, Patterson Y. Rhesus macaques with the highest level of vaccine induced IFN-gamma producing cells leads to lower viral set-points following challenge with SIV239. Vaccine. 2006;24:4498–4502. doi: 10.1016/j.vaccine.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Blake K, O’Connell S. Virus Culture. In: Harper DR, editor. Virology Labfax. BIOS Sceintific Publishers; Oxford, UK: 1993. pp. 81–122. [Google Scholar]
- Broussard SR, Staprans SI, White R, Whitehead EM, Feinberg MB. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J Virol. 2001;75:2262–2275. doi: 10.1128/JVI.75.5.2262-2275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L. The paradox of simian immunodeficiency virus infection in sooty mangabeys: active viral replication without disease progression. Frontiers in Bioscience. 2004;9:521–539. doi: 10.2741/1123. [DOI] [PubMed] [Google Scholar]
- Connor RI, Montefiori DC, Binley JM, Moore JP, Bonhoeffer S, Gettie A, Fenamore EA, Sheridan KE, Ho DD, Dailey PF, Marx PA. Temporal analysis of virus replication, immune responses and efficacy in rhesus macaques immunized with a live attenuated simian immunodeficiency virus vaccine. J Virol. 1998;72:7501–7509. doi: 10.1128/jvi.72.9.7501-7509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MP, Ribeiro RM, Zhang L, Wilson DP, Perelson AS. Understanding the mechanisms and limitations of immune control of HIV. Immunological Reviews. 2007;216:164–175. doi: 10.1111/j.1600-065X.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- De Rozières S, Mathiason CK, Rolston MR, Chatterji U, Hoover EA, Elder JH. Characterization of a highly pathogenic molecular clone of Feline Immunodeficiency Virus Clade C. J Virol. 2004;78:8971–8982. doi: 10.1128/JVI.78.17.8971-8982.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douek DC, Picker LJ, Koup RA. T Cell Dynamics in HIV-1 Infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- Dua N, Reubel G, Moore PF, Higgins J, Pedersen NC. An experimental study of primary feline immunodeficiency virus infection in cats and a historical comparison to acute simian and human immunodeficiency virus diseases. Vet Immuno Immunopathol. 1994;43:337–355. doi: 10.1016/0165-2427(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Egberink H, Horzinek MC. Animal immunodeficiency viruses. Vet Microbiol. 1992;33:311–331. doi: 10.1016/0378-1135(92)90059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea M, Smith DM, Liu L, May S, Tenorio AR, Ignacio CC, Landay A, Haubrich R. Determinants of CD4+ T Cell Recovery during suppressive antiretroviral therapy: Association of immune activation, T Cell maturation markers and cellular HIV-1 DNA. JID. 2006;194:29–37. doi: 10.1086/504718. [DOI] [PubMed] [Google Scholar]
- Goldstein S, Ourmanov I, Brown CR, Plishka R, Buckler-White A, Byrum R, Hirsch VM. Plateau levels of viremia correlate with the degree of CD4+ -T-Cell loss in simian immunodeficiency virus SIVagm-infected pigtailed macaques: variable pathogenicity of natural SIVagm isolates. J Virol. 2005;79:5253–5162. doi: 10.1128/JVI.79.8.5153-5162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Leutenegger CM, Dean GA, Steckbeck JD, Cole KS, Sparger EE. Vaccination with attenuated feline immunodeficiency virus proviral DNA vaccine expressing interferon gamma. J Virol. 2007;81:465–473. doi: 10.1128/JVI.00815-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hel Z, McGhee RR, Mestecky J. HIV infection: first battle decides the war. TRENDS in Immunology. 2006;27:274–281. doi: 10.1016/j.it.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Hosie MJ, Flynn N, Rigby MA, Cannon C, Dunsford T, Mackay NA, Argyle DA, Williet BJ, Miyazawa T, Onions TE, Jarrett O, Neil JC. DNA Vaccination affords significant protection against feline immunodeficiency virus infection without inducing detectable antiviral antibodies. J Virol. 1988;72:7310–7319. doi: 10.1128/jvi.72.9.7310-7319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost S, Turelli P, Mangeat B, Protzer U, Trono D. Induction of antiviral cytidine deaminases does not explain the inhibition of hepatitis B virus replication by interferons. J Virol. 2007;81:10588–10596. doi: 10.1128/JVI.02489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada M, Tsukamoto T, Yamamoto H, Takeda A, Igarashi H, Watkins DI, Matano T. Long-term control of simian immunodeficiency virus replication with central memory CD4+ T-cell preservation after nonsterile protection by a cytotoxic T-lymphocyte-based vaccine. J Virol. 2007;81:5202–5211. doi: 10.1128/JVI.02881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld C, Ploquin MJ-Y, Pandrea I, Faye A, Onanga R, Apetrei C, Poaty-Mavoungou V, Rouguet P, Estaquier J, Montara L, Desoutter J-F, Butor C, Le Grand R, Roques P, Simon R, Barré-Sinoussi F, Diop OM, Müller-Trutwin MC. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115:1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthels Altes H, de Boer R, Boerlijst M. Role of avidity and breadth of the D4 T cell response in progression to AIDS. Proc R Soc B. 2006;273:1697–1704. doi: 10.1098/rspb.2006.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner AA, Veazy RS. Current concepts in AIDS pathogenesis: Insights form the SIV/Macaque model. Annu Rev Med. 2007;58:461–476. doi: 10.1146/annurev.med.58.082405.094316. [DOI] [PubMed] [Google Scholar]
- Langlois ARC, Desrosiers RC, Lewis MG, Kewalramoan VN, Littman DR, Shou JY, Manson K, Wyaand MS, Bolognesi DP, Montefiori DC. Neutralizing antibodies in sera from Macaques immunized with attenuated simian immunodeficiency virus. J Virol. 1998;72:6950–6955. doi: 10.1128/jvi.72.8.6950-6955.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lama J. The physiological relevance of CD4 receptor down-modulation during HIV infection. Curr HIV Res. 2003;1:167–184. doi: 10.2174/1570162033485276. [DOI] [PubMed] [Google Scholar]
- Leutenegger CM, Mislin CN, Sigrist B, Ehrengruber MU, Hofmann-Lehmann R, Lutz H. Quantitative real-time PCR for the measurement of feline cytokine mRNA. Vet Immunol Immunopathol. 1999;71:291–305. doi: 10.1016/S0165-2427(99)00100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima VD, Hogg RS, Harrigan PR, Moore D, Yip B, Wood E, Montaner JS. Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS. 2007;21:685–692. doi: 10.1097/QAD.0b013e32802ef30c. [DOI] [PubMed] [Google Scholar]
- Lindwasser OW, Chaudhuri R, Bonifacino JS. Mechanisms of CD4 downregulation by the nef and vpu proteins of primate immunodeficiency viruses. Curr Mol Med. 2007;7:171–184. doi: 10.2174/156652407780059177. [DOI] [PubMed] [Google Scholar]
- Loffredo JT, Burwitz BJ, Rakasz EG, Spencer SP, Stephany JJ, Vela JPG, Martin SR, Reed J, Piaskowki SM, Furlott J, Weisgrau KL, Rodrigues DS, Soma T, Napoé G, Friedrich TC, Wilson NA, Kallas EG, Watkins DI. The antiviral efficacy of simian immunodeficiency virus-specific CD8+ T Cells is unrelated to epitope specificity and is abrogated by viral escape. J Virol. 2007;81:2624–2634. doi: 10.1128/JVI.01912-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl JN, Regoes RR, Garber DA, Feinberg MB. Estimating the effectiveness of SIV specific CD8+ T cells from the dynamics of viral immune escape. J Virol. 2007;81:11982–11991. doi: 10.1128/JVI.00946-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ. HIV Vaccines. Annu Rev Immunol. 2006;24:227–255. doi: 10.1146/annurev.immunol.24.021605.090605. [DOI] [PubMed] [Google Scholar]
- Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, Kingsley LA, Todd JA, Saah AJ, Detels R, Phair JP, Rinaldo CR. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV infection. Ann Int Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Genescà M, Abel K, Montefiori D, Forthal D, Bost D, Li J, Favre D, McCune FM. Antiviral antibodies are necessary for control of simian immunodeficiency virus replication. J Virol. 2007;81:5024–5035. doi: 10.1128/JVI.02444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa T, Furuya T, Itagaki S, Tohya T, Takahashi E, Mikami T. Establishment of a feline T-lymphoblastoid cell line highly sensitive for replication of feline immunodeficiency virus. Arch Virol. 1989;108:131–135. doi: 10.1007/BF01313750. [DOI] [PubMed] [Google Scholar]
- Overbaugh J, Luciw PA, Hoover EA. Models for AIDS pathogenesis: simian immunodeficiency virus, simian-human immunodeficiency virus and feline immunodeficiency virus infections. AIDS. 1997;11:S47–S54. [PubMed] [Google Scholar]
- Pedersen NC, Leutenegger CM, Woo J, Higgins J. Virulence differences between two field isolates of feline immunodeficiency virus (FIV-APetaluma and FIV-CPGammar) in young adult specific pathogen free cats. Vet Immunol Immunopathol. 2001;79:53–67. doi: 10.1016/s0165-2427(01)00252-5. [DOI] [PubMed] [Google Scholar]
- Peretz Y, Alter G, Boisvert M-P, Hatzakis G, Tsoukas CM, Bernard NF. Human immunodeficiency virus (HIV)-specific gamma interferon secretion directed against all expressed HIV genes: relationship to rate of CD4 decline. J Virol. 2005;79:4908–4917. doi: 10.1128/JVI.79.8.4908-4917.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss M, Ross HA, Painter SL, Holley DC, TerWee JA, VandeWoude S, Rodrigo A. Feline lentivirus evolution in cross-species infection reveals extensive G-to-A mutation and selection on key residues in the viral polymerase. J Virol. 2006;80:2728–2737. doi: 10.1128/JVI.80.6.2728-2737.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss M, Idoine A, Ross HA, TerWee JA, VandeWoude S, Rodrigo A. Recombination in feline lentiviral genomes during experimental cross-species infection. Virology. 2007;359:146–151. doi: 10.1016/j.virol.2006.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RA, Sinka KJ, Molesworth AM, Evans BG, Allardice GM. Survival after diagnosis of AIDS among adults resident in the United Kingdom in the era of multiple therapies. Commun Dis Public Health. 2000;3:188–194. [PubMed] [Google Scholar]
- Quinnan GV, Jr, Yu X-F, Lewis MG, Zhang PF, Sutter G, Silera P, Dong M, Choudhary A, Sarkis PTN, Bouma P, Zhang Z, Montefiori DC, VanCott TC, Broder CC. Protection of rhesus monkeys against infection with minimally pathogenic simian-human immunodeficiency virus: Correlations with neutralizing antibodies and cytotoxic T cells. J Virol. 2005;79:3358–3369. doi: 10.1128/JVI.79.6.3358-3369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey-Cuille MA, Berthier JL, Bomsel-Demontoy MC, Chaduc Y, Montagnier L, Hovenessian AG, Chakrabarti LA. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J Virol. 1998;72:3872–3876. doi: 10.1128/jvi.72.5.3872-3886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodŕiguez B, Sethi AK, Cheruvu VK, Mackay W, Bosch RJ, Kitahata M, Boswell SL, Matthews WC, Bansberg DR, Martin J, Whalen CC, Sieg S, Tadavalli S, Deeks SG, Lederman MM. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296:1498–1506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- Rossio JL, Esser MT, Suryanaryana F, Schneider DK, Bess JW, Jr, Vasquez GM, Wiltrout TA, Chertova E, rimes MK, Sattentau Q, Arthur LO, Henderson LE, Lifson JD. Inactivation of human immunodeficiency virus type 1 with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, Johnson RP, McClure HM, Manson KH, Wyand MS, Kuroda MJ, Lifton MA, Khunkhun RS, McEvers KJ, Gillis J, Piatak M, Lifson JD, Grosschupff G, Racz P, Tenner-Racz K, Rieber EP, Kuus-Reichel K, Gelman RS, Letvin NL, Montefiori DC, Rupprecht RM, Desrosiers RC, Reimann KA. Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge after live attenuated SIVmac239Δ3-vaccinated rhesus macaques. J Virol. 2005;79:8131–8141. doi: 10.1128/JVI.79.13.8131-8141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebelink KH, Chu IH, Rimmelzwaan GF, Weijer K, van Herwijnen R, Knell P, Egberink HF, Bosch ML, Osterhaus AD. Feline immunodeficiency virus (FIV) infection in the cat as a model for HIV infection in man: FIV-induced impairment of immune function. AIDS Res Hum Retroviruses. 1990;6:1373–1378. doi: 10.1089/aid.1990.6.1373. [DOI] [PubMed] [Google Scholar]
- Singh DK, Liu A, Sheffer D, Mackay GA, Smith M, Dhillon S, Hegde R, Jia F, Adany I, Narayan O. A noninfectious simian/human Immunodeficiency virus DNA vaccine that protects macaques against AIDS. J Virol. 2005;79:3419–3428. doi: 10.1128/JVI.79.6.3419-3428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova N, Troyer JL, Schissler J, TerWee J, Poss M, VandeWoude S. Feline lentiviruses demonstrate differences in receptor repertoire and envelope structural elements. Virology. 2005;342:60–76. doi: 10.1016/j.virol.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Sondgeroth K, Leutenegger C, VandeWoude S. Development and validation of puma (Felis concolor) cytokine and lentivirus real-time PCR detection systems. Vet Immunol Immunopathol. 2005;104:205–213. doi: 10.1016/j.vetimm.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Stebbings RJ, Almond NM, Stott J, Berry N, Wade-Evans AM, Hull R, Lines J, Silvera P, Sangster R, Corcoran T, Rose J, Walker KB. Mechanisms of protection induced by attenuated simian immunodeficiency virus V. No evidence for lymphocyte-regulated cytokine responses upon rechallenge. Virology. 2002;296:338–353. doi: 10.1006/viro.2002.1379. [DOI] [PubMed] [Google Scholar]
- Stolte-Leeb N, Sauermann U, Norley S, Fagrouch Z, Heeney H, Franz M, Hunsmann G, Stahl-Hennig C. Sustained conservation of CD4+ cells in multiprotein triple modality immunized rhesus macaques after intrarectal challenge with simian immunodeficiency virus. Viral Immunol. 2006;19:448–457. doi: 10.1089/vim.2006.19.448. [DOI] [PubMed] [Google Scholar]
- Sun Y, Schmitz JE, Buzby AP, Barker BR, Rao SS, Xu L, Yang ZY, Mascla JR, Nabel GJ, Letvin NL. Virus-specific cellular immune correlates of survival in vaccinated monkeys after simian immunodeficiency virus challenge. J Virol. 2006;80:10950–10956. doi: 10.1128/JVI.01458-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerWee JA, Yactor JK, Sondgeroth KS, VandeWoude S. Puma Lentivirus is controlled in domestic cats after mucosal exposure in the absence of conventional indicators of immunity. J Virol. 2005;79:2797–2806. doi: 10.1128/JVI.79.5.2797-2806.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeWoude S, Hageman CA, O’Brien SJ, Hoover EA. Nonpathogenic lion and puma lentiviruses impart resistance to superinfection by virulent feline immunodeficiency virus. JAIDS. 2002;29:1–10. doi: 10.1097/00126334-200201010-00001. [DOI] [PubMed] [Google Scholar]
- VandeWoude S, Apetrei C. Going Wild: Lessons from naturally occurring T-lymphotropic lentiviruses. Clin Microbiol Rev. 2006;19:728–762. doi: 10.1128/CMR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet L, Monceaux W, Petit F, Ho Tsong Fang R, Cumont MC, Hurtel B, Estaquier J. Death of CD4+ T cells from lymph nodes during primary IVmac251 infection predicts the rate of AIDS progression. J Immunol. 2006;177:6685–94. doi: 10.4049/jimmunol.177.10.6685. [DOI] [PubMed] [Google Scholar]
- Yates A, Stark J, Klein N, Antia R, Callard R. Understanding the slow depletion of memory CD4+ T-cells in HIV infection. PLoS Med. 2007;4:e177. doi: 10.1371/journal.pmed.0040177. [DOI] [PMC free article] [PubMed] [Google Scholar]


