Abstract
The photoreceptor-specific nuclear receptor (PNR; Nr2e3) is a transcription factor important for retinal development. We report here the identification and expression analysis of the avian Nr2e3. Nr2e3 mRNA is expressed in the photoreceptor layer of the neural retina during early stages of chick embryogenesis. Its temporal expression is distinct from that of a related nuclear receptor, Tlx. Chick Nr2e3 recognizes and binds to the same target DNA sequence as its vertebrate orthologs. Functional assays revealed that chick Nr2e3 acts as a transcriptional repressor. Our results suggest that Nr2e3 plays a common role in retinal development in vertebrates.
Keywords: Nuclear receptor, Transcription factor, Eye development, Chicken
Introduction
Transcription factor proteins regulate gene expression by binding to specific DNA sequences in regulatory regions of the gene. Nuclear receptors have a unique modular structure, with a characteristic ligand-binding domain in addition to their DNA-binding domain (Mangelsdorf et al. 1995). They are receptors for lipophilic molecules such as steroid and thyroid hormones, vitamins A and D, and at the same time, they function as transcription factors. Many nuclear receptors are known to regulate cell fate determination. However, ligands have not been identified for a substantial number of nuclear receptors and members of this class are called orphan receptors (Benoit et al. 2006).
Photoreceptor-specific nuclear receptor (PNR; Nr2e3) is one of these orphan receptors, whose expression is restricted almost exclusively to the photoreceptor layer of the neural retina (Kobayashi et al. 1999; Chen et al. 2005; Kitambi and Hauptmann 2007; Martinez-De Luna and El-Hodiri 2007; McIlvain and Knox 2007). Mutations of this gene have been implicated in several forms of retinal disorder (Gerber et al. 2000 and references in Chen et al. 2005). Detailed analyses of its function have been conducted using various model vertebrate systems and suggested that Nr2e3 is important in the development and maintenance of rod photoreceptor cells (Chen et al. 2005; McIlvain and Knox 2007; Oh et al. 2008).
Chick embryos have long been a favorite model for performing functional analyses of transcription factor proteins. It allows observation of retinal development in vivo with relative ease and the availability of gene transfer methods such as electrophoresis is an additional advantage (Stern 2005). Identification of the chick Nr2e3 gene is, thus, a useful tool to further these studies.
We used a cDNA library from embryonic day 8 (E8) chick retina to identify an avian Nr2e3 clone. The mRNA expression of this chick Nr2e3 was found to be restricted in the neural retina. We analyzed its expression during a developmental time course along with a related transcription factor, Tlx. Structurally, the chick Nr2e3 shares an asparagine substitution in the P box of its DNA-binding domain with its vertebrate orthologs in contrast to the acidic amino acid residue present in other nuclear receptor family members. Accordingly, the Nr2e3 proteins show the same DNA-binding preferences, suggesting that they regulate a similar set of target genes. Transcription assays further confirmed that chick Nr2e3 function as a transcriptional repressor.
Materials and methods
RNA extraction
Fertilized chicken eggs were obtained from Okada Farm (Nara, Japan) and incubated at 35°C for up to 14 days. The embryos were harvested, tissues were dissected, and total RNA was extracted using ISOGEN (Nippon Gene, Toyama, Japan) following the manufacturer’s protocol.
Identification of the chicken Nr2e3 ortholog
Using total RNA extracted from the neural retina of E8 chick embryos, mRNA was purified with Oligotex™-dT30 <Super> Kit (Roche). A lambda phage cDNA library was constructed using the Lambda System for cDNA Cloning (Life Technologies) and Gigapack III Gold Packaging extract (Stratagene). A polymerase chain reaction fragment corresponding to full-length human Nr2e3 (amino acids 8 to poly(A)+ region) was amplified from a human PNR (Nr2e3) plasmid construct using primers NMO70 (5′-TCTGATGAGCTCCACAGTGGCTGC-3′) and T3 (5′-GCAATTAACCCTCACTAAAGGG-3′) (Kobayashi et al. 1999). The DNA fragment was gel-purified, labeled with alpha-32P-dCTP using Megaprime DNA Labeling System (Amersham Pharmacia) and used as a probe to screen 0.8 × 105 independent clones in the E8 retinal library using an ExAssist helper phage (Stratagene). Twenty-seven positive clones were identified and further analyzed in a second screening using the same probe. Fifteen strongly positive clones were selected for DNA sequencing and three potential chicken Nr2e3 clones were identified. The most 5′ end, which corresponded to the region between the DNA- and ligand-binding domain of the potential chicken Nr2e3 was amplified using primers KMO40 (5′-TAGACAGCATTGAGCTGGACGCCGA-3′) and KMO22 (5′-CGTGCTGAGGTCTCGTAGACGTT-3′) and another screening was performed. Independent clones of 2.0 × 105 were screened and nine positive clones were identified. After a second screening using the same probe, three positive clones were identified and DNA sequence was determined using an ALF express sequencing machine (Amersham Pharmacia).
Northern blotting and in situ hybridization
A 0.75-kb cDNA fragment of chicken Nr2e3 [nucleotides 177–915] was amplified using primers KMO6 (5′-CCAGTGGACAAGGCACACCGCAAC-3′) and KMO22 and labeled with digoxigenin (Boehringer; for Nr2e3 probe), or amplified using KMOcT5 (5′-ATCCAGGCTTTACAGGAGGTTGTGG-3′) and KMOcT3 (5′-TCCGAAACGACAGGGTTGTGTAGG-3′) and labeled with alpha-32P-dCTP (for Tlx probe). Gel electrophoresis, membrane transfer, and hybridization conditions were as described (Kobayashi et al. 1999). A nylon membrane blot containing 10 μg of each total RNA was prepared. The 18S and 28S rRNA on the membrane was visualized by 5% methylene blue in 10% acetic acid and used as loading control. After hybridization with the Nr2e3 probe, the signals were detected using an ECL Detection System (Amersham Pharmacia). Subsequently, the blot was rehybridized with a Tlx probe and the signal was visualized on X-ray film. In situ hybridization of frozen tissue sections was performed as described (Kobayashi et al. 1999) using a 0.75-kb digoxygenin-labeled antisense RNA probe.
DNA-binding assays
The chick Nr2e3 protein was translated from a pCMX cNr2e3 expression vector (Kobayashi et al. 1999) using T7 RNA polymerase and a TNT® coupled rabbit reticulocyte in vitro translation kit (Promega). Probe-labeling and gel shift assay was performed as described (Kobayashi et al. 1999). The sequences of double-stranded probes used for the assays are indicated in the figure with 5′-agct hangovers.
Transactivation assays
Expression plasmids for the GAL4–Nr2e3 fusion protein were obtained by ligating cDNA fragments encoding chick (Arg 92 to stop codon) or human (Met 113 to stop codon) Nr2e3 ligand-binding domain into a cytomegalovirus promoter-driven plasmid pCMX-GAL4 (Gerber et al. 2000). CV-1 cells were used for transfection assays in 12-well tissue culture plates by calcium phosphate precipitation (Umesono and Evans 1989). Cells were maintained in Dulbecco’s modified Eagle's medium (DMEM) supplemented with 10% bovine calf serum (BCS). Transfection mixtures contained 100 ng of receptor expression plasmid, 250 ng of MH100 × 4-tk-luc reporter plasmid and 500 ng of pCMX-βGAL as control for transfection efficiency. Cells were exposed to the precipitate for 6–8 h, washed, and treated with phenol red-free DMEM, 10% charcoal–resin-treated BCS, before harvesting and assaying for luciferase and β-galactosidase activity. All points were performed in triplicate and repeated at least twice in independent experiments with variations of less than 10%.
Results and discussion
Identification of the chicken Nr2e3 gene
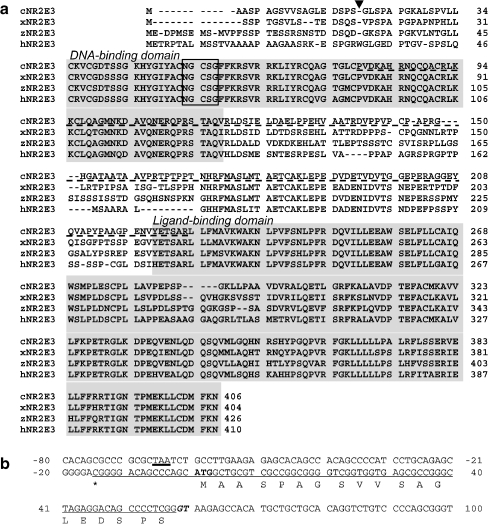
In vertebrates including humans and mice, the Nr2e3 gene is known to be exclusively expressed in the neural retina (Kobayashi et al. 1999; Chen et al. 2005; Kitambi and Hauptmann 2007; Martinez-De Luna and El-Hodiri 2007; McIlvain and Knox 2007). Therefore, to identify a chick Nr2e3 cDNA, we constructed a phage cDNA library from E8 chick neural retina. A total of 0.3 × 106 independent clones were screened and six clones that showed a high homology to mammalian Nr2e3 were identified. The deduced amino acid sequence of chick Nr2e3 showed 70% homology to its mammalian counterparts, with even more similarity to the amphibian Nr2e3 (Fig. 1a; Kobayashi et al. 1999; Chen et al. 2005; Martinez-De Luna and El-Hodiri 2007). Three other clones, which showed relatively high hybridization signals to the Nr2e3 probe, were found to encode Nr2e1 (Tlx) and Nr2f (COUP-TF) family genes, which are most closely related to Nr2e3 among the nuclear receptor superfamily (Benoit et al. 2006). Translation of the Nr2e3 protein is assumed to initiate from the first ATG codon found in the cloned mRNA because an in-frame stop codon (TAA) was found 80 bp upstream of the initiation codon in Gallus gallus whole genome shotgun sequence pqd72h09.b1 (accession number 140842551; Fig. 1b). In addition, a putative splice donor sequence (gt) for the first intron is found at the same position where the first intron is located in the zebrafish Nr2e3 gene (Chen et al. 2005).
Fig. 1.
a Sequence alignment of chicken, Xenopus, zebrafish, and human Nr2e3. DNA- and ligand-binding domains are shaded. The position of the first intron and the P box are indicated by an arrowhead and a box. The dashed underline indicates the region used as a probe for northern and in situ hybridization analyses. b Primary structure of the 5′ region of the chicken Nr2e3 gene. The sequence found in cDNA clone(s) is underlined with the 5′ end indicated by asterisk. The first ATG is in bold, with an in-frame upstream stop codon (bold-underlined). The splice donor sequence (GT) is shown in italics
Nr2e3 mRNA is expressed in photoreceptor cells of neural retina
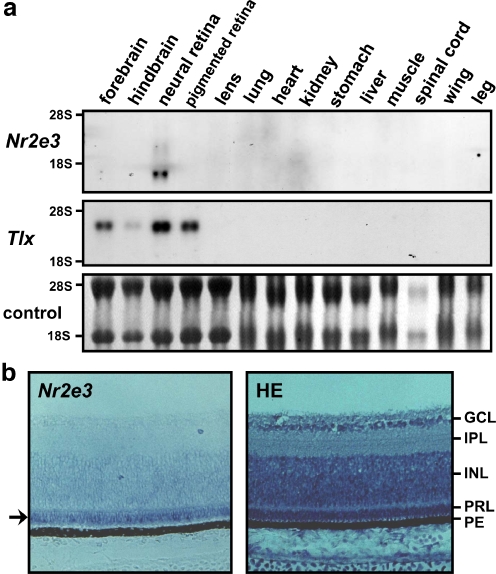
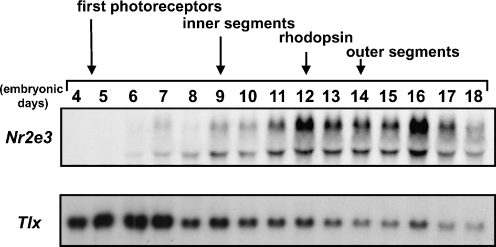
Total RNA was extracted from different tissues of E8 chick embryos and the pattern of Nr2e3 mRNA expression was analyzed. Nr2e3 expression was observed only in the neural retina among the organs examined, while mRNA of the related Tlx (Yu et al. 2000) was also expressed in the brain and pigmented retina (Fig. 2a). Using RNA from E14 chick embryos, the restricted expression of Nr2e3 in the neural retina was confirmed (data not shown). In situ hybridization of E14 chick retina sections revealed that Nr2e3 expression is restricted to the photoreceptor layer of neural retina (Fig. 2b). These results confirm that the clone we identified in chicken is indeed an ortholog of the mammalian Nr2e3. To elucidate the potential role of Nr2e3 in retinal development, its mRNA expression was examined using RNA extracted from neural retina at different stages (Fig. 3). The expression of Nr2e3 increased in the later stages of embryogenesis, while the expression of Tlx was the highest in the early stages (E5 to E7) and gradually declined (Fig. 3). Considering that Nr2e3 is almost exclusively expressed in the photoreceptor layer, it is interesting that the expression of Nr2e3 mRNA gradually increases as photoreceptor cells develop.
Fig. 2.
Expression of Nr2e3 mRNA in chicken. a Northern hybridization analyses of mRNA from E8 chick embryo tissues/organs using the chicken Nr2e3 or Tlx cDNA as probes. All lanes contain 10 μg of total RNA. For Nr2e3, strong signal was detected around 1.7 kb and relatively weak signal of 2.6 kb. The 18S and 28S rRNA were used as a loading control. b In situ hybridization of E14 chick embryo retina sections with digoxigenin-labeled Nr2e3 RNA probe. The arrow indicates Nr2e3 signal in the photoreceptor layer. An adjacent section was stained with hematoxylin–eosin (HE) to help visualize different cell layers. GCL Ganglion cell layer, IPL inner plexiform layer, INL inner nuclear layer, PRL photoreceptor layer, PE pigment epithelium
Fig. 3.
Temporal expression of Nr2e3 and Tlx mRNA in early retinal development. Northern hybridization analyses of mRNA from the neural retina of chick embryos at different stages of development using the chick Nr2e3 or Tlx cDNA as a probe. All lanes contain 10 μg of total RNA. Approximate timing of events in eye development are indicated
DNA-binding specificity of Nr2e3
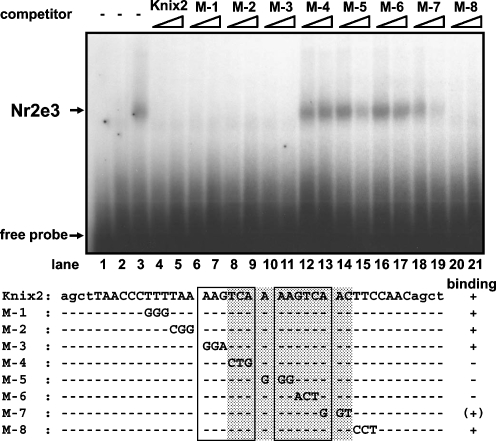
The first amino acid in the Nr2e3 P box of its DNA-binding domain (Umesono and Evans 1989), which is critical for recognition of target DNA sequence, is an asparagine (N) as is the case for other Nr2e3 orthologs (Fig. 1a; Kobayashi et al. 1999; Chen et al. 2005). This is a unique feature among nuclear receptors and it has been shown that mammalian and zebrafish Nr2e3 bind to a tandem repeat of AAGTCA motifs separated by one nucleotide (Kobayashi et al. 1999; Chen et al. 2005). To examine the binding specificity of chick Nr2e3 protein, a gel mobility shift assay was performed using a probe to which mammalian Nr2e3 protein was shown to bind. Chick Nr2e3 showed the same binding preferences with its mammalian counterpart (Fig. 4). Competition experiments further revealed that nucleotides important for recognition by mammalian Nr2e3 were also conserved in chick Nr2e3 and necessary for its target DNA specificity (Fig. 4).
Fig. 4.
In vitro DNA binding preference by Nr2e3 protein. A gel mobility shift assay was performed. Lane 1 is probe only, and mock cell extract was added in lane 2. Lane 3 shows a specific protein–DNA complex formed when Nr2e3 protein is added. Lanes 4–11 and 19–21 show that Nr2e3 bound to labeled Kni x2 probe can be blocked by competition by addition of excess mutant cold probes shown below. Competitor probes are indicated above, with ten-fold excess added in lanes 4, 6, 8, 10, 12, 14, 16, 18, 20, and 50-fold excess in lanes 5, 7, 9, 11, 13, 15, 17, 19, 21. Shaded region indicates the nucleotide sequence shown to be important for recognition by Nr2e3 protein. The tandem repeat of AAGTCA motifs in Kni x2 probe are boxed
Function of Nr2e3
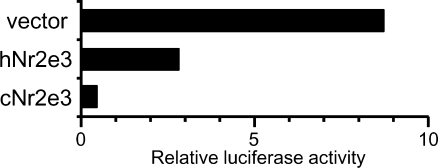
In earlier studies, mammalian Nr2e3 was shown to repress transcription of its target genes (Gerber et al. 2000; Chen et al. 2005). To test the transcriptional activity of the chick Nr2e3, a plasmid coding for the GAL4 DNA-binding domain fused to the Nr2e3 ligand-binding domain was constructed and cotransfected with a reporter plasmid controlled by a promoter containing GAL4-binding sites (Fig. 5). Along with human Nr2e3, we confirmed that the chick Nr2e3 acts as a transcriptional repressor (Fig. 5).
Fig. 5.
Nr2e3 protein represses transcription. Transient transfection assays in CV-1 cells show that the chick Nr2e3 can repress the activity of a luciferase reporter gene to a greater extent than human Nr2e3
The role of the Nr2e3 gene in vertebrates has been widely analyzed (Gerber et al. 2000; Chen et al. 2005; McIlvain and Knox 2007; Oh et al. 2008) and suggests a common function for Nr2e3 in those species. Recently, a Nr2e3 ortholog was reported in the genome of the gray, short-tailed opossum (Monodelphis domestica) published as the first metatherian (‘marsupial’) species to be sequenced (GenBank accession number XM_001371384). In addition, putative Nr2e3 orthologs have been found in Caenorhabditis elegans (Kobayashi et al. 1999; Wightman et al. 2005) and sea urchin Strongylocentrotus purpuratus (GenBank accession number XP_780706). The C. elegans counterpart, FAX-1, also contains an asparagine in its P box and has shown to be important for axon pathfinding and specification of neuron identities (Wightman et al. 2005). Considering the restricted expression of Nr2e3 in the vertebrate neural retina, it is interesting to investigate the function of Nr2e3 in those species that do not have apparent organ comparable to a vertebrate eye. Potential molecules that may modulate Nr2e3 function have also been reported, adding a tool to investigate the function of Nr2e3 (Wolkenberg et al. 2006).
Regarding factors that might control Nr2e3 expression, a Maf protein Nrl has been suggested for its regulation (Oh et al. 2008). Although Nrl is not found in chicken, another Maf protein, L-Maf has been found to be expressed in rod cells in the later stages (Ochi et al. 2004). It is possible that L-Maf or other Maf protein(s) may regulate Nr2e3 expression in chicken.
In conclusion, we succeeded in identifying a chicken ortholog of the Nr2e3 gene, whose mRNA expression is found only in the photoreceptor layer of the neural retina. During chick development, Nr2e3 expression was high in the later stages (E12 to E17) while the expression of closely related Tlx gene was high in the early stages (E5 to E7). The binding specificity of the avian Nr2e3 protein shares the same preferences as its vertebrate orthologs and also functions as a transcriptional repressor. Our results suggest that Nr2e3 has a common function in retinal development that is shared between avians and mammals.
Acknowledgements
The authors would like to acknowledge that this project was initiated and mainly conducted under the direct supervision of late Professor Kazuhiko Umesono at Kyoto University. The authors thank Dr. Yoshihiko Umesono for sharing unpublished results and Dr. Shin-ichiro Takezawa for plasmid construction. This work was supported in part by grants from the Human Frontier Science Program; the Research for the Future Program of the Japan Society for the Promotion of Science; Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan; and the Foundation for Nara Institute of Science and Technology.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
The sequence reported in this paper has been deposited in the GenBank database [accession no. AY053465 (chicken Nr2e3)].
References
- Benoit G, Cooney A, Giguere V, Ingraham H, Lazar M, Muscat G, Perlmann T, Renaud JP, Schwabe J, Sladek F, Tsai MJ, Laudet V. International Union of Pharmacology. LXVI. Orphan nuclear receptors. Pharmacol Rev. 2006;58:798–836. doi: 10.1124/pr.58.4.10. [DOI] [PubMed] [Google Scholar]
- Chen J, Rattner A, Nathans J. The rod photoreceptor-specific nuclear receptor Nr2e3 represses transcription of multiple cone-specific genes. J Neurosci. 2005;25:118–129. doi: 10.1523/JNEUROSCI.3571-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber S, Rozet JM, Takezawa S-I, dos Santos LC, Lopes L, Gribouval O, Penet C, Perrault I, Ducroq D, Souied E, Jeanpierre M, Romana S, Frezal J, Ferraz F, Yu-Umesono R, Munnich A, Kaplan J. The photoreceptor cell-specific nuclear receptor gene (PNR) accounts for retinitis pigmentosa in the Crypto-Jews from Portugal (Marranos), survivors from the Spanish inquisition. Hum Genet. 2000;107:276–284. doi: 10.1007/s004390000350. [DOI] [PubMed] [Google Scholar]
- Kitambi SS, Hauptmann G. The zebrafish orphan nuclear receptor genes Nr2e1 and Nr2e3 are expressed in developing eye and forebrain. Gene Expr Patterns. 2007;7:521–528. doi: 10.1016/j.modgep.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Takezawa S, Hara K, Yu RT, Umesono Y, Agata K, Taniwaki M, Yasuda K, Umesono K. Identification of a photoreceptor cell-specific nuclear receptor. Proc Natl Acad Sci U S A. 1999;96:4814–4819. doi: 10.1073/pnas.96.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily—the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-De Luna RI, El-Hodiri HM. The Xenopus ortholog of the nuclear hormone receptor Nr2e3 is primarily expressed in developing photoreceptors. Int J Dev Biol. 2007;51:235–239. doi: 10.1387/ijdb.062236rm. [DOI] [PubMed] [Google Scholar]
- McIlvain VA, Knox BE. Nr2e3 and Nrl can reprogram retinal precursors to the rod fate in Xenopus retina. Dev Dyn. 2007;236:1970–1979. doi: 10.1002/dvdy.21128. [DOI] [PubMed] [Google Scholar]
- Ochi H, Sakagami K, Ishii A, Morita N, Nishiuchi M, Ogino H, Yasuda K. Temporal expression of L-Maf and RaxL in developing chicken retina are arranged into mosaic pattern. Gene Expr Patterns. 2004;4:489–494. doi: 10.1016/j.modgep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Oh EC, Cheng H, Hao H, Jia L, Khan NW, Swaroop A (2008) Rod differentiation factor NRL activates the expression of nuclear receptor NR2E3 to suppress the development of cone photoreceptors. Brain Res: doi:10.1016/j.brainres.2008.01.028 [DOI] [PMC free article] [PubMed]
- Stern CD. The chick: a great model system becomes even greater. Dev Cell. 2005;8:9–17. doi: 10.1016/j.devcel.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Umesono K, Evans RM. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989;57:1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Wightman B, Ebert B, Carmean N, Weber K, Clever S. The C. elegans nuclear receptor gene fax-1 and homeobox gene unc-42 coordinate interneuron identity by regulating the expression of glutamate receptor subunits and other neuron-specific genes. Dev Biol. 2005;287:74–85. doi: 10.1016/j.ydbio.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Wolkenberg SE, Zhao ZJ, Kapitskaya A, Webber AL, Petrukhin K, Tang YS, Dean DC, Hartman GD, Lindsley CW. Identification of potent agonists of photoreceptor-specific nuclear receptor (Nr2e3) and preparation of a radioligand. Bioorg Med Chem Lett. 2006;16:5001–5004. doi: 10.1016/j.bmcl.2006.07.056. [DOI] [PubMed] [Google Scholar]
- Yu RT, Chiang M-Y, Tanabe T, Kobayashi M, Yasuda K, Evans RM, Umesono K. The orphan nuclear receptor Tlx regulates Pax2 and is essential for vision. Proc Natl Acad Sci USA. 2000;97:2621–2625. doi: 10.1073/pnas.050566897. [DOI] [PMC free article] [PubMed] [Google Scholar]