Abstract
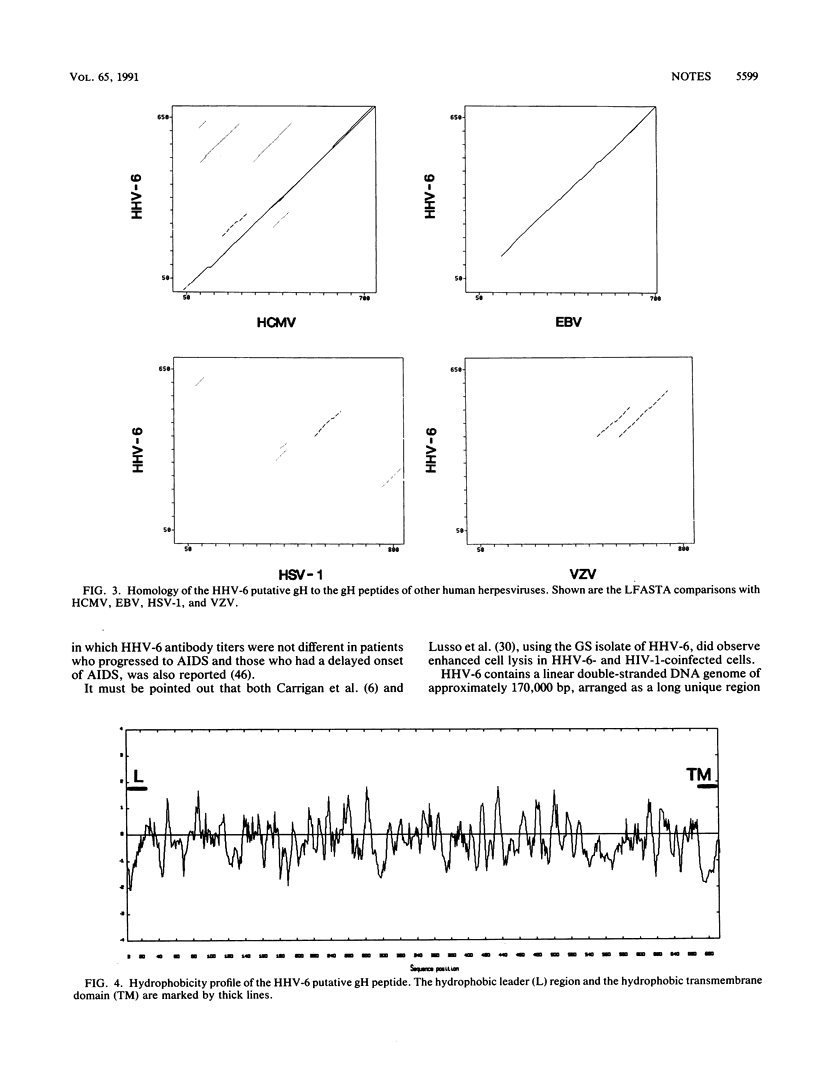
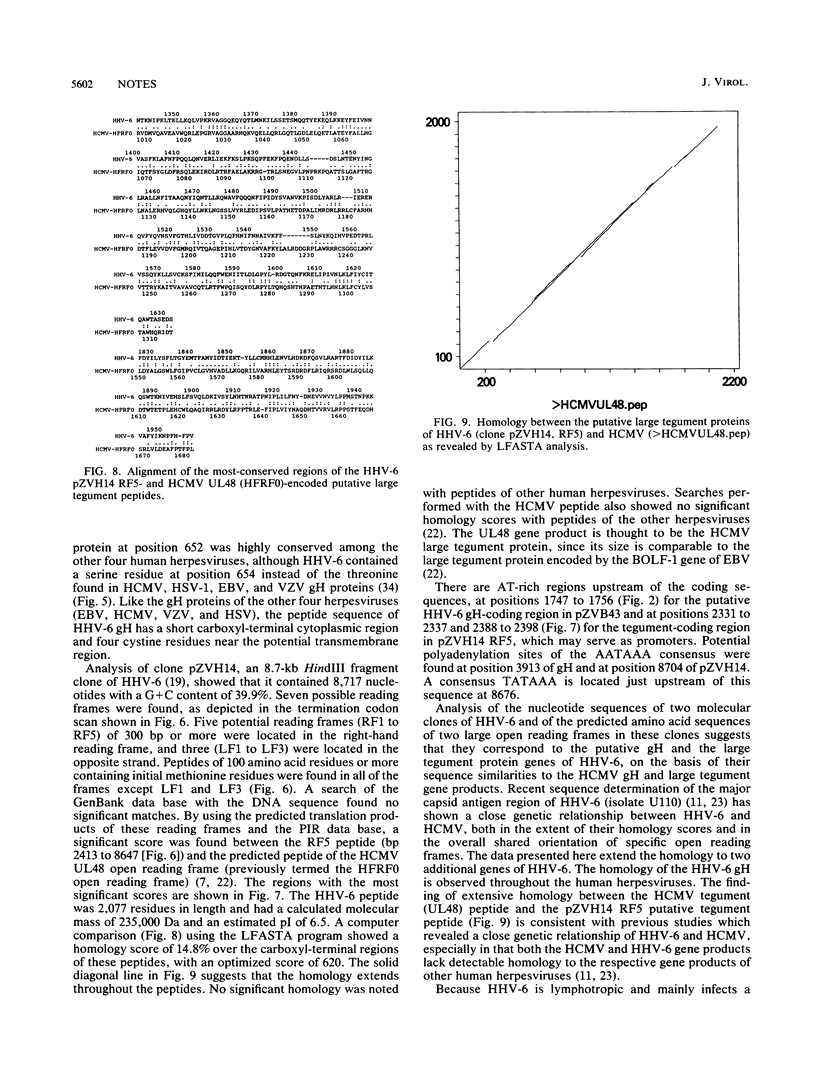
Determination of the nucleotide sequences of two molecular clones of human herpesvirus 6 (HHV-6) (strain GS) and comparison with those of human cytomegalovirus (HCMV) has allowed the identification of the genes for the glycoprotein H (gH) and the putative large tegument protein of HHV-6. Two molecular clones of fragments of HHV-6, the BamHI-G fragment (7,981 bp) of the clone termed pZVB43 and a HindIII fragment (8,717 bp) of the clone termed pZVH14, represent approximately 10% of the HHV-6 genome (16,689). An open reading frame within the BamHI-G fragment was designated the gH gene of HHV-6 because of the extensive sequence similarity of its predicted product (79,549 Da) to the HCMV gH gene product. The predicted product (239,589 Da) of an open reading frame within clone pZVH14 showed homology to the predicted product of the proposed gene of HCMV representing the large tegument protein. Computer analyses indicated a closer relationship of the predicted peptides of these HHV-6 genes to those of HCMV than to those of the other human herpesviruses Epstein-Barr virus, herpes simplex virus type 1, and varicella-zoster virus. The gH gene was more conserved among the human herpesvirus group, while significant sequence similarity of the tegument gene could be found only with that of HCMV. The data reported here with one conserved gene (gH) and a more divergent gene (tegument) support previous reports that HHV-6 and HCMV are more closely related to each other than to the other well-characterized human herpesviruses.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablashi D. V., Lusso P., Hung C. L., Salahuddin S. Z., Josephs S. F., Llana T., Kramarsky B., Biberfeld P., Markham P. D., Gallo R. C. Utilization of human hematopoietic cell lines for the propagation and characterization of HBLV (human herpesvirus 6). Int J Cancer. 1988 Nov 15;42(5):787–791. doi: 10.1002/ijc.2910420526. [DOI] [PubMed] [Google Scholar]
- Ablashi D. V., Salahuddin S. Z., Josephs S. F., Balachandran N., Krueger G. R., Gallo R. C. Human herpesvirus-6 (HHV-6) (short review). In Vivo. 1991 May-Jun;5(3):193–199. [PubMed] [Google Scholar]
- Ablashi D. V., Salahuddin S. Z., Josephs S. F., Imam F., Lusso P., Gallo R. C., Hung C., Lemp J., Markham P. D. HBLV (or HHV-6) in human cell lines. Nature. 1987 Sep 17;329(6136):207–207. doi: 10.1038/329207a0. [DOI] [PubMed] [Google Scholar]
- Biberfeld P., Kramarsky B., Salahuddin S. Z., Gallo R. C. Ultrastructural characterization of a new human B lymphotropic DNA virus (human herpesvirus 6) isolated from patients with lymphoproliferative disease. J Natl Cancer Inst. 1987 Nov;79(5):933–941. [PubMed] [Google Scholar]
- Carrigan D. R., Knox K. K., Tapper M. A. Suppression of human immunodeficiency virus type 1 replication by human herpesvirus-6. J Infect Dis. 1990 Oct;162(4):844–851. doi: 10.1093/infdis/162.4.844. [DOI] [PubMed] [Google Scholar]
- Chee M. S., Bankier A. T., Beck S., Bohni R., Brown C. M., Cerny R., Horsnell T., Hutchison C. A., 3rd, Kouzarides T., Martignetti J. A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Chee M. S., Lawrence G. L., Barrell B. G. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J Gen Virol. 1989 May;70(Pt 5):1151–1160. doi: 10.1099/0022-1317-70-5-1151. [DOI] [PubMed] [Google Scholar]
- Cranage M. P., Smith G. L., Bell S. E., Hart H., Brown C., Bankier A. T., Tomlinson P., Barrell B. G., Minson T. C. Identification and expression of a human cytomegalovirus glycoprotein with homology to the Epstein-Barr virus BXLF2 product, varicella-zoster virus gpIII, and herpes simplex virus type 1 glycoprotein H. J Virol. 1988 Apr;62(4):1416–1422. doi: 10.1128/jvi.62.4.1416-1422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. G., Kenney S. C., Kamine J., Pagano J. S., Huang E. S. Immediate-early gene region of human cytomegalovirus trans-activates the promoter of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8642–8646. doi: 10.1073/pnas.84.23.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou S., Gompels U. A., Craxton M. A., Honess R. W., Ward K. DNA homology between a novel human herpesvirus (HHV-6) and human cytomegalovirus. Lancet. 1988 Jan 2;1(8575-6):63–64. doi: 10.1016/s0140-6736(88)91049-5. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Lusso P., Schachter F., Josephs S. F., Rappaport J., Negro F., Gallo R. C., Wong-Staal F. Human herpes virus-6 increases HIV-1 expression in co-infected T cells via nuclear factors binding to the HIV-1 enhancer. EMBO J. 1989 Oct;8(10):3019–3027. doi: 10.1002/j.1460-2075.1989.tb08452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D. G., Barker W. C., Hunt L. T. The protein identification resource (PIR). Nucleic Acids Res. 1986 Jan 10;14(1):11–15. doi: 10.1093/nar/14.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat R. T., Wood C., Balachandran N. Transactivation of human immunodeficiency virus promoter by human herpesvirus 6. J Virol. 1989 Feb;63(2):970–973. doi: 10.1128/jvi.63.2.970-973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett R. F., Gledhill S., Qureshi F., Crae S. H., Madhok R., Brown I., Evans I., Krajewski A., O'Brien C. J., Cartwright R. A. Identification of human herpesvirus 6-specific DNA sequences in two patients with non-Hodgkin's lymphoma. Leukemia. 1988 Aug;2(8):496–502. [PubMed] [Google Scholar]
- Josephs S. F., Ablashi D. V., Salahuddin S. Z., Kramarsky B., Franza B. R., Jr, Pellett P., Buchbinder A., Memon S., Wong-Staal F., Gallo R. C. Molecular studies of HHV-6. J Virol Methods. 1988 Sep;21(1-4):179–190. doi: 10.1016/0166-0934(88)90064-x. [DOI] [PubMed] [Google Scholar]
- Josephs S. F., Buchbinder A., Streicher H. Z., Ablashi D. V., Salahuddin S. Z., Guo H. G., Wong-Staal F., Cossman J., Raffeld M., Sundeen J. Detection of human B-lymphotropic virus (human herpesvirus 6) sequences in B cell lymphoma tissues of three patients. Leukemia. 1988 Mar;2(3):132–135. [PubMed] [Google Scholar]
- Josephs S. F., Salahuddin S. Z., Ablashi D. V., Schachter F., Wong-Staal F., Gallo R. C. Genomic analysis of the human B-lymphotropic virus (HBLV). Science. 1986 Oct 31;234(4776):601–603. doi: 10.1126/science.3020691. [DOI] [PubMed] [Google Scholar]
- Kenney S., Kamine J., Markovitz D., Fenrick R., Pagano J. An Epstein-Barr virus immediate-early gene product trans-activates gene expression from the human immunodeficiency virus long terminal repeat. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1652–1656. doi: 10.1073/pnas.85.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M., Harada H., Takahashi M., Tanaka A., Hayashi M., Nonoyama M., Josephs S. F., Buchbinder A., Schachter F., Ablashi D. V. A repeat sequence, GGGTTA, is shared by DNA of human herpesvirus 6 and Marek's disease virus. J Virol. 1988 Dec;62(12):4824–4827. doi: 10.1128/jvi.62.12.4824-4827.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Large-scale rearrangement of homologous regions in the genomes of HCMV and EBV. Virology. 1987 Apr;157(2):397–413. doi: 10.1016/0042-6822(87)90282-0. [DOI] [PubMed] [Google Scholar]
- Lawrence G. L., Chee M., Craxton M. A., Gompels U. A., Honess R. W., Barrell B. G. Human herpesvirus 6 is closely related to human cytomegalovirus. J Virol. 1990 Jan;64(1):287–299. doi: 10.1128/jvi.64.1.287-299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Ferro F., Greenspan D., Lennette E. T. Frequent isolation of HHV-6 from saliva and high seroprevalence of the virus in the population. Lancet. 1990 May 5;335(8697):1047–1050. doi: 10.1016/0140-6736(90)92628-u. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Landay A., Lennette E. T. Human herpesvirus 6 inhibits human immunodeficiency virus type 1 replication in cell culture. J Clin Microbiol. 1990 Oct;28(10):2362–2364. doi: 10.1128/jcm.28.10.2362-2364.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lopez C., Pellett P., Stewart J., Goldsmith C., Sanderlin K., Black J., Warfield D., Feorino P. Characteristics of human herpesvirus-6. J Infect Dis. 1988 Jun;157(6):1271–1273. doi: 10.1093/infdis/157.6.1271. [DOI] [PubMed] [Google Scholar]
- Lusso P., De Maria A., Malnati M., Lori F., DeRocco S. E., Baseler M., Gallo R. C. Induction of CD4 and susceptibility to HIV-1 infection in human CD8+ T lymphocytes by human herpesvirus 6. Nature. 1991 Feb 7;349(6309):533–535. doi: 10.1038/349533a0. [DOI] [PubMed] [Google Scholar]
- Lusso P., Ensoli B., Markham P. D., Ablashi D. V., Salahuddin S. Z., Tschachler E., Wong-Staal F., Gallo R. C. Productive dual infection of human CD4+ T lymphocytes by HIV-1 and HHV-6. Nature. 1989 Jan 26;337(6205):370–373. doi: 10.1038/337370a0. [DOI] [PubMed] [Google Scholar]
- Lusso P., Markham P. D., Tschachler E., di Marzo Veronese F., Salahuddin S. Z., Ablashi D. V., Pahwa S., Krohn K., Gallo R. C. In vitro cellular tropism of human B-lymphotropic virus (human herpesvirus-6). J Exp Med. 1988 May 1;167(5):1659–1670. doi: 10.1084/jem.167.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. E., Thomson B. J., Honess R. W., Craxton M. A., Gompels U. A., Liu M. Y., Littler E., Arrand J. R., Teo I., Jones M. D. The genome of human herpesvirus 6: maps of unit-length and concatemeric genomes for nine restriction endonucleases. J Gen Virol. 1991 Jan;72(Pt 1):157–168. doi: 10.1099/0022-1317-72-1-157. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Davison A. J. DNA sequence of the herpes simplex virus type 1 gene encoding glycoprotein gH, and identification of homologues in the genomes of varicella-zoster virus and Epstein-Barr virus. Nucleic Acids Res. 1986 May 27;14(10):4281–4292. doi: 10.1093/nar/14.10.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Hayward G. S., Pitha P. M. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7408–7412. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Niederman J. C., Liu C. R., Kaplan M. H., Brown N. A. Clinical and serological features of human herpesvirus-6 infection in three adults. Lancet. 1988 Oct 8;2(8615):817–819. doi: 10.1016/s0140-6736(88)92783-3. [DOI] [PubMed] [Google Scholar]
- Pietroboni G. R., Harnett G. B., Farr T. J., Bucens M. R. Human herpes virus type 6 (HHV-6) and its in vitro effect on human immunodeficiency virus (HIV). J Clin Pathol. 1988 Dec;41(12):1310–1312. doi: 10.1136/jcp.41.12.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando R. F., Pellett P. E., Luciw P. A., Bohan C. A., Srinivasan A. Transactivation of human immunodeficiency virus by herpesviruses. Oncogene. 1987 Mar;1(1):13–18. [PubMed] [Google Scholar]
- Rice A. P., Mathews M. B. Trans-activation of the human immunodeficiency virus long terminal repeat sequences, expressed in an adenovirus vector, by the adenovirus E1A 13S protein. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4200–4204. doi: 10.1073/pnas.85.12.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin S. Z., Ablashi D. V., Markham P. D., Josephs S. F., Sturzenegger S., Kaplan M., Halligan G., Biberfeld P., Wong-Staal F., Kramarsky B. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986 Oct 31;234(4776):596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Josephs S. F., Dukovich M., Peffer N., Wong-Staal F., Greene W. C. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987 Dec 11;238(4833):1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- Spira T. J., Bozeman L. H., Sanderlin K. C., Warfield D. T., Feorino P. M., Holman R. C., Kaplan J. E., Fishbein D. B., Lopez C. Lack of correlation between human herpesvirus-6 infection and the course of human immunodeficiency virus infection. J Infect Dis. 1990 Mar;161(3):567–570. doi: 10.1093/infdis/161.3.567. [DOI] [PubMed] [Google Scholar]
- Steeper T. A., Horwitz C. A., Ablashi D. V., Salahuddin S. Z., Saxinger C., Saltzman R., Schwartz B. The spectrum of clinical and laboratory findings resulting from human herpesvirus-6 (HHV-6) in patients with mononucleosis-like illnesses not resulting from Epstein-Barr virus or cytomegalovirus. Am J Clin Pathol. 1990 Jun;93(6):776–783. doi: 10.1093/ajcp/93.6.776. [DOI] [PubMed] [Google Scholar]
- Suga S., Yoshikawa T., Asano Y., Yazaki T., Hirata S. Human herpesvirus-6 infection (exanthem subitum) without rash. Pediatrics. 1989 Jun;83(6):1003–1006. [PubMed] [Google Scholar]
- Takahashi K., Sonoda S., Kawakami K., Miyata K., Oki T., Nagata T., Okuno T., Kamanishi K. Human herpesvirus 6 and exanthem subitum. Lancet. 1988 Jun 25;1(8600):1463–1463. doi: 10.1016/s0140-6736(88)92275-1. [DOI] [PubMed] [Google Scholar]
- Tedder R. S., Briggs M., Cameron C. H., Honess R., Robertson D., Whittle H. A novel lymphotropic herpesvirus. Lancet. 1987 Aug 15;2(8555):390–392. doi: 10.1016/s0140-6736(87)92404-4. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Okuno T., Shiraki K., Takahashi M., Kondo T., Asano Y., Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988 May 14;1(8594):1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]



