Abstract
MYCN activation, mainly by gene amplification, is one of the most frequent molecular events in neuroblastoma (NB) oncogenesis, and is associated with increased malignancy and decreased neuronal differentiation propensity. The frequency of concomitant loss of heterozygosity at the 1p36.3 locus, which harbours the p53 anti-oncogene homologue TP73, indicates that MYCN and p73 alterations may cooperate in the pathogenesis of NB. We have previously shown that p73 isoforms are deregulated in NB tumours and that TAp73 co-operates synergistically with p53 for apoptosis of NB cells, whereas ΔNp73 activates the expression of neuronal differentiation genes such as BTG2. Herein, using both ectopic expression and RNA interference-mediated silencing of p73 in MYCN amplified NB cells, we show that p73α isoforms inhibit MYCN expression at both transcript and protein levels, in spite of transactivator effects on MYCN promoter. To explain this paradox, we found that TAp73α exerts negative post-transcriptional effects leading to reduced MYCN mRNA stability. RNA immunoprecipitation experiments suggest that this dominant inhibitory post-transcriptional effect could be due to an interaction between the p73 protein and MYCN mRNA, a hypothesis also raised for the regulation of certain genes by the p53 protein.
INTRODUCTION
Neuroblastomas (NB), paediatric cancers developing from peripheral sympathetic neurons, frequently display amplification of the MYCN proto-oncogene, which strongly correlates with advanced disease stage and poor outcome (1,2). The MYCN protein was shown to play a crucial role in NB tumorigenesis, notably by promoting cell proliferation and impeding differentiation (3).
NB also recurrently present loss of heterozygosity on chromosome 1p36, a region altered in many types of cancers (4–6). The p53 anti-oncogene homologue TP73 was discovered at this locus (7). Although the remaining TP73 allele is only exceptionally mutated, we and others have shown that quantitative and qualitative p73 alterations contribute to the pathogenesis of NB, albeit not as a classical tumour suppressor (8,9).
In addition to structural homology, p73 also shares functional homology with p53, TAp73 being notably capable to transactivate many p53-responsive genes. However, in p73, the C-terminal region is, unlike that of p53, composed of a Sterile Alpha Motif (SAM) domain and a transactivation inhibitor (TI) domain (Supplementary Figure S1). The SAM domains are widespread among eukaryotes and bacteria (reviewed in 10), have been identified in nearly 1000 proteins and are potentially involved in a large spectrum of biological processes. SAM domains were notably implicated in protein–protein interactions, and the SMAUG-like SAM domains have recently been showed to bind specific RNA sequences named SAM response elements (SRE) (11). The SAM domain of p73 was shown to play a transcription inhibitory role by preventing, in concert with the TI domain, the accessibility of p300/CBP to the activation domain of p73 (12). It could also mediate other biological functions through its capacity to interact with lipid membranes (13).
The p73 gene encodes multiple isoforms, due to alternative promoter usage and mRNA splicing (Supplementary Figure S1). TAp73 isoforms, harbouring a transactivating domain (TA), are known to transactivate p53 responsive genes and to induce apoptosis and growth arrest, whereas N-terminal truncated ΔNp73 isoforms, lacking the TA domain, can act as dominant-negative towards p53 and TAp73 (14,15). Splicing at the 3′ end of p73 transcripts gives rise to C-terminal protein variants containing (isoforms α) or not (isoforms β and others) the SAM domain and the TI region.
Interferon-γ was shown to activate p73, which in turn activates caspase-1 in Hela cells (16). Additionally, interferon-γ is known to down-regulate MYCN mRNA in NB cells treated with retinoic acid (17). Moreover, TAp73-induced differentiation of mouse neuroblastoma cells was associated with MYCN protein down-regulation (18). These observations suggested that p73 can inhibit MYCN expression in NB cells, without indicating whether the regulation operates at the transcriptional or post-transcriptional level. The present study aims to answer to these questions and to elucidate the molecular mechanism involved.
MATERIALS AND METHODS
Constructs and siRNA
The plasmid constructs pcDNA-TAp73α, pcDNA-TAp73β, pcDNA-ΔNp73α and pcDNA-ΔNp73β, obtained by cloning the various cDNA into the pcDNA3-Neo vector (Invitrogen), were kindly provided by Dr Mourad Kaghad (Sanofi-Aventis Recherche, Labège, France). The empty pcDNA3-Neo vector was used as a control. The pGL3-NMYC-Luc reporter construct, containing the proximal MYCN promoter cloned in front of the firefly luciferase cDNA, as described (19), was a kind gift from Drs Marianne Kim and William Carroll (Mount Sinai School of Medicine, New York). The pGL3-Luc vector (Promega) was used as a control. For gene silencing experiments, we used small interfering RNA (siRNA), consisting of chemically synthesized 21-mer oligoribonucleotide duplexes (QIAGEN). As a non-silencing control, we designed a siRNA (sequence: 5'-AAAGACGGTGGTCATTACCTAGT-3') targeting the RFP mRNA (accession number AF168419, encoding coral red fluorescence protein); the TAp73 siRNA, targeting exon 3 of the human TP73 gene, was as previously described (20).
Cell culture, transient transfections and chemical treatments
The human neuroblastoma cell lines SH-SY5Y, SK-N-AS and IMR32 were purchased from the European Collection of Cell Cultures (ECACC, Wiltshire, UK). The Kelly and LAN-1 cells were respectively provided by Dr Jean-Philippe Deslys (Commissariat à l’Energie Atomique, Fontenay-Aux-Roses, France) and Dr Nicole Gross (Paediatric Oncology Research, Lausanne, Switzerland). IMR32, Kelly and LAN-1 cells were grown in RPMI medium supplemented with 10 mM Hepes, 10% fetal calf serum and 10 mg/ml of gentamicine (GIBCO). Other cell lines were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 10 mg/ml of gentamicine. Cells were maintained at 37°C in a humidified incubator with 5% CO2.
Plasmids were transfected into cells plated in 6 well plates using Lipofectamine 2000 (Invitrogen) and siRNA using Oligofectamine (Invitrogen), according to the manufacturer's instructions. For luciferase assays, plasmids and siRNA were cotransfected using Lipofectamine 2000. For plasmids transfections, cells were plated at a density of 106 cells/well, were transfected the following day with 4 µg of plasmid and harvested 2 days later for protein or RNA analyses. For siRNA transfections, cells were plated at a density of 2 × 105 cells/well for Kelly or LAN-1 cells and 5 × 105 cells/well for SH-SY5Y cells. Cells were transfected the following day with siRNAs, at a final concentration of 200 nM, and collected 3 days later for protein and RNA analyses.
To measure MYCN transcripts half-life, cells were treated 48 h after transfection with 5 µM of Actinomycin D to block mRNA synthesis, then harvested after 0.5, 1, 2, 3 and 6 h of treatment for RNA analysis, as described below.
At least three independent series of transfections, with duplicates for each condition, were performed and analysed.
RNA extraction, reverse transcription and polymerase reaction
Total RNA was extracted and treated with DNase I using the RNeasy mini kit (QIAGEN), according to the manufacturer's instructions. RNA (1 µg) was converted into single strand complementary DNA (cDNA) using superscript II reverse transcriptase (Invitrogen). Reactions in which the reverse transcriptase was omitted (RT-) were used as negative controls. Quantitative reverse transcription-polymerase chain reaction (qRT–PCR) was performed in a final volume of 25 µl containing 25 ng of cDNA or RT- as templates, 10 pmol of each primer and 12.5 µl of SYBR®-Green master mix (Applied Biosystems). The sets of primers used were: for MYCN: 5′-CACCCTGAGCGATTCAGATGA-3′ and 5′-CCGGGACCCAGGGCT-3′; for c-MYC: 5′-CCACCAGCAGCGACTCTGA-3′ and 5′-GCAGAAGGTGATCCAGACTC-3′; for TAp73: 5′-GCACCACGTTTGAGCACCTCT-3′ and 5'-GCAGATTGAACTGGGCCATGA-3′; for ΔNp73: 5′-CAAACGGCCCGCATGTTCCC-3′ and 5′-TTGAACTGGGCCGTGGCGAG-3′; for p73Δex2-3: 5′-TGCAGGCCCAGTTCAATCTGC-3′ and 5′-TGCGTGTTGGAGGGGATGACA-3′; for GAPDH: 5′-AGCTCACTGGCATGGCCTTC-3′ and 5′-ACGCCTGCTTCACCACCTTC-3′; for 18S rRNA: 5′-CGGCTACCACATCCAAGGAA-3′ and 5′-GCTGGAATTACCGCGGCT-3′. The amplification reactions were performed and monitored in real-time using the ABI Prism 7000 Sequence Detection system (Applied Biosystems). MYCN and p73 mRNA levels were normalized between samples using GAPDH mRNA and 18S rRNA as references. For semi-quantitative RT–PCR analyses, 12.5 ng of cDNA or RT-controls were amplified in a final volume of 10 µl containing 0.25 units of AmpliTaq Gold DNA polymerase (Roche Applied Science) containing 1.75 mM MgCl2, 200 µM of each nucleotide and 0.5 µM of each primer. We used this method to determine the relative transcript levels of α and β isoforms of p73, with primers flanking exon 13 of the TP73 gene, which is spliced out in β but not α isoforms of p73: forward: 5′-CCGACCCCAGCCTCGTCAG-3′; reverse: 5′-CTGAGCCGCCGATGGA-3′. Amplification of GAPDH was used as a control for normalization. PCR reactions consisted of an initial denaturation step of 10 min at 94°C, then 30 cycles (TP73) or 20 cycles (GAPDH) of 30 s at 94°C, 30 s at 60°C and 1 min 10 s at 72°C using a PTC-100 thermocycler (MJ-Research). The number of cycles was chosen to be in the exponential phase of the amplification. PCR products were subjected to electrophoresis in 1.8% agarose gels, then stained for 30 min with SYBR®-Green I (Molecular Probes). Gels were visualized using a Fujifilm FLA-3000 scanner (FUJI), and the amplified fragments were quantified with the IMAGE J software.
Western blot
Immunoblotting was carried out as described previously (21). Thirty to 50 µg of total protein were separated by 4–12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred onto nitrocellulose filters. After saturation, the membranes were incubated with primary antibodies diluted in Tris-buffered saline (TBS) containing 0.1% Tween-20 and 5% skim milk. The primary antibodies used, all mouse monoclonal, except for p73 (rabbit polyclonal), were: anti-p73 (a kind gift from Dr Mourad Kaghad, Sanofi-Aventis Recherche, Labège, France), anti-p73β (Ab-3, Oncogene Research), anti-MYCN (Ab-1, Oncogene Research), anti-p53 (DO-7, DAKO), all 4 at a dilution of 1/1000 and anti-β-actin (Chemicon) at a dilution of 1/5000 as a loading control. After washes in TBS-tween, incubation with a secondary horseradish peroxidase coupled anti-mouse or anti-rabbit antibody (at a dilution of 1/2000 except for β-actin, 1/10 000) and subsequent washes, protein bands were revealed using ECL reagent (GE Healthcare), except for p73 for which we used Immobilon Western (Millipore).
Luciferase reporter assays
For luciferase reporter assays, cells were seeded in triplicates in 12-well plates at a density of 3 × 105 cells/well. The following day, cells were co-transfected with 0.5 µg of either control or MYCN promoter containing pGL3-Luc reporter constructs, together with 1 µg of expression vectors or 200 pmol of siRNA, using Lipofectamine 2000, as described above. Twenty-four hours after transfection, cells were lysed with passive lysis buffer (100 µl/well), provided with the ‘Luciferase assay kit’ (Promega). Firefly luciferase activity was measured according to the manufacturer's protocol using Microlumat LB96P luminometer (EG&G Berthold Instruments).
Chromatin and RNA immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed as described in one of our recent reports (22), using 4 µg of mouse monoclonal antibody raised against p73 (Ab2, Calbiochem). Non-precipitated chromatin and chromatin precipitated in the presence of a pre-immune mouse IgG were used as positive and negative controls, respectively. The precipitated chromatin was then purified by phenol–chloroform extraction and ethanol precipitation. Interaction between the p73 protein and MYCN gene promoter region was searched by PCR using the following pairs of primers: ATGCTGCTGCTGGACAGAG (−1237) and TGATTCCAAACTGTTGAAGGG (−1009); CCCTCGTAGCTCGCACTTATT (−668) and AAAGAAGGGTAGTCCGAAGGT (−518); CGGGTGTGTCAGATTTTTCAGT (−61) and TGCTCGGCTCCAACACAGTTC (+64), numbered nucleotide referring to the transcription start site. PCR conditions (28 cycles, using 1/40th of the immunoprecipitated DNA as a template) and SYBR®-Green I-stained agarose gel analysis were performed as described in the semi-quantitative RT–PCR section.
For RNA immunoprecipitation (RNA IP), 107 cells were lysed with 300 µl polysome lysis buffer (KCl 100 mM, MgCl2 5 mM, Hepes pH7 10 mM, Nonidet P-40 0.5%) containing RNase and protease inhibitors. One hundred microlitres of cellular extracts were kept as non-immunoprecipitated input control. Fifty microlitre of protein G Dynabeads (Invitrogen) were pre-incubated for 1 h in 200 µl of IP buffer containing 50 mM of Tris pH7.4, 150 mM NaCl, 5 mM MgCl2 and 0.05% Nonidet p-40. We then added 4 µg of control or p73 antibodies (see the ChIP section above) and homogenized the mix overnight at 4°C. One hundred microlitres of cellular extracts were added to each control and p73 antibody containing mixes, and immunoprecipitations were carried out for 2 h at room temperature under constant homogenization. The beads were washed 6 times using IP buffer (see above), then treated with 30 µg of proteinase K diluted in 100 µl of IP buffer containing 0.1% SDS. Immunoprecipitated RNA was then purified using phenol/chloroform extraction followed by ethanol precipitation. Interaction between the p73 protein and MYCN transcript was searched by quantitative reverse transcription-polymerase chain reaction (qRT–PCR) analysis, as described above.
RESULTS
p73 inhibits MYCN expression at both mRNA and protein levels
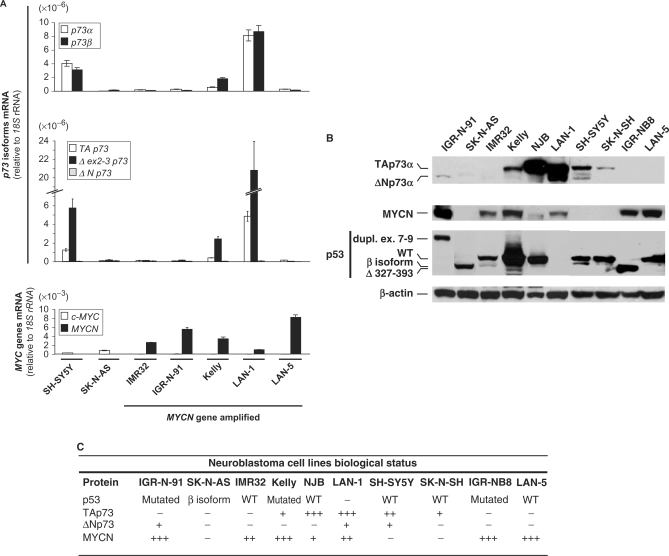
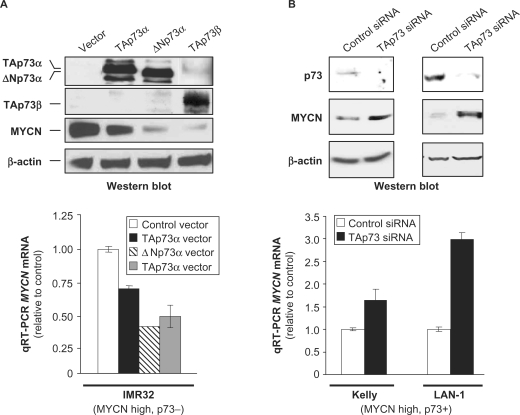
In order to investigate the impact of p73 on MYCN expression in neuroblastoma (NB) cells, we selected, among a panel of NB cell lines, MYCN amplified and overexpressing cells with different p73 status. Most of the tested NB cell lines overexpress MYCN, at the RNA and protein levels (Figure 1A and B). Among MYCN overexpressing cell lines, some, such as IMR32, lack endogenous p73 expression, while others, such as Kelly and LAN-1, express moderate or high levels of p73 (Figure 1A and B). We thus first used IMR32 cells to test the effect of ectopic p73 expression on MYCN levels. Transfection of a TAp73α expression vector in these cells led to a clear inhibition of MYCN protein levels (Figure 2A, top). The N- and C-terminal-truncated ΔNp73α and TAp73β isoforms produced a similar or even more pronounced inhibitory effect on MYCN protein levels. Consistent with the ectopic expression data, RNA-interference-mediated silencing of endogenous TAp73 in Kelly and LAN-1 cell lines was associated with significant MYCN protein levels increase (Figure 2B, top). The TP73 siRNA used in these and subsequent experiments targets exon 3 of the gene (20) and thus specifically inhibit the TA isoforms, both α and β. At the RNA level, the Kelly and LAN-1 cells express the TA (minor) and Δex2-3 (major) TP73 5′ isoforms, and both the α and β 3′ TP73 splice variants, in equal proportions for LAN-1 (Figure 1A). However, western blot analyses indicate that, at the protein level, these two cell lines express TAp73α but not the C-terminal truncated TAp73β isoform (Figure 2B, top), as confirmed with a p73β-specific antibody that did not detect any band in these cells (data not shown). Although a long exposure of the western blots indicated that LAN-1 cells express both TA and ΔNp73 protein isoforms (Figure 1B), another blot with shorter exposure showed that these cells express mainly the full-length TAp73 protein isoform (Figure 2B, top). A distinct siRNA targeting TAp73, although having a lower silencing efficiency than the previous one, led to similar effects on MYCN levels but at a lesser extent (Supplementary Figure S2). Based on these observations, we conclude that the observed siRNA effects are due to depletion of the TAp73α isoform. Considering the differences in the p53 status of the studied cell lines (Figure 1), the inhibitory effect of p73 on MYCN protein levels appears to be independent of p53. Indeed, as examples, p73 isoforms repress MYCN levels in IMR32 cells, which express wild type p53, and in LAN-1 and IGR-N-91 cells, which respectively lack p53 protein expression or express a mutant p53 protein.
Figure 1.
(A) Analysis of TP73, c-MYC and MYCN genes transcript levels in a panel of human neuroblastoma (NB) cell lines. Semi-quantitative reverse transcription-polymerase chain reaction (RT–PCR) was used to monitor the levels of p73α and p73β transcripts. TAp73, Δex2-3p73, ΔNp73, c-MYC and MYCN transcript levels were evaluated using real-time quantitative RT–PCR (qRT–PCR). (B) Western blot analysis of p73, MYCN and p53 protein levels and status (isoform, mutation) in human NB cell lines. For p53, WT refers to the wild-type protein; dupl. ex 7–9 and Δ 373–393 correspond to mutations with duplication of exons 7–9 and deletion of aminoacids 327–393, respectively; the β isoform is a recently described p53 variant generated by alternative splicing in a cryptic site causing a premature stop codon in the C-terminal region. (C) Table summarizing the expression and isoform/mutational status of p73, MYCN and p53 protein in this series of human NB cell lines.
Figure 2.
P73 inhibits MYCN expression at the protein and RNA levels in human neuroblastoma cells. (A) Effects of ectopic expression of p73 isoforms (top panel) on MYCN protein (top panel) and MYCN RNA (bottom panel) levels in IMR32 neuroblastoma (NB) cells. Protein levels were monitored by western blotting (top panel). As the first p73 antibody used preferentially recognizes the α isoform, western blot analysis was also performed with an anti-p73β antibody. Ectopic expression of TAp73β also resulted in a moderate up-regulation of p73α, likely as a consequence of transactivation of genes such as that encoding E2F1 that is known to activate TAp73α expression. MYCN transcript levels were evaluated in matching RNA samples using real-time quantitative RT–PCR (qRT–PCR, bottom panel). (B) Similar analyses were performed to evaluate the impact of small interfering RNA (siRNA)-mediated depletion of endogenous TAp73 on MYCN protein (top panel) and MYCN RNA (bottom panel) levels in Kelly and LAN-1 NB cells. Plotted qRT–PCR values are the means ± SEM of three replicates.
We then examined the impact of p73 on MYCN mRNA levels. Ectopic expression of p73α and β isoforms in IMR32 cells was associated with a decrease in MYCN mRNA ranging from 1.5- to 2.5-fold (Figure 2A, bottom), indicating that the regulation occurs at the transcriptional or post-transcriptional level. In agreement with these observations, silencing of endogenous TAp73α in Kelly and LAN-1 cells, respectively led to a 1.5- and 3-fold increase in MYCN mRNA levels (Figure 2B, bottom). Ectopic expression of both TA- and ΔN-p73α isoforms also caused a decrease in MYCN mRNA levels in IGR-N-91, another MYCN amplified NB cell line that lacks endogenous p73 (Supplementary Figure S3). Therefore, although to a different extent, TAp73α, ΔNp73α and TAp73β all inhibited MYCN mRNA accumulation.
p73α, but not β isoforms, lead to activation of MYCN gene transcription without binding to its promoter
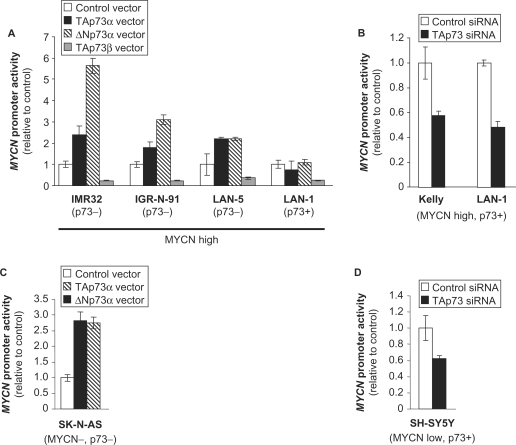
Based on its inhibitory effects on MYCN mRNA levels, we then tested the impact of p73 on MYCN gene transcription. Surprisingly, ectopic expression of TAp73α in IMR-32 cells led to a >2-fold increase in the activity of a MYCN promoter reporter construct (Figure 3A). Interestingly, the ΔNp73α isoform yielded an even stronger (>5-fold) MYCN promoter activation, indicating that the N-terminal transcriptional activator (TA) domain of p73 is not required and that p73α leads to MYCN gene transactivation via an alternative mechanism (Figure 3A). On the contrary, ectopic TAp73β expression caused a 4-fold inhibition of MYCN promoter activity in IMR32 cells (Figure 3A). MYCN promoter activation in response to ectopic expression of TAp73α and ΔNp73α was similarly observed in IGR-N-91 and LAN-5 cells exhibiting little or no detectable endogenous p73 protein, respectively, whereas no significant effects were observed in LAN-1 cells expressing high endogenous p73 (Figures 1A and 3). However, TAp73β retained its inhibitory effects on MYCN promoter in LAN-1 like in the other cell lines (Figure 3A). Reciprocally, depletion of endogenous TAp73α in Kelly and LAN-1 cells led to an approximately 2-fold decrease in MYCN promoter activity (Figure 3B).
Figure 3.
P73α but not β isoforms lead to activation of MYCN gene transcription in human neuroblastoma cells. Effects of ectopic expression of p73 isoforms (A and C) or small interfering RNA (siRNA)-mediated depletion of endogenous TAp73 (B and D) on MYCN gene promoter activity in neuroblastoma cell lines. Values represent the mean Luciferase reporter gene activity ± SEM of three replicates.
Our observations demonstrate that, although p73α isoforms lead to MYCN promoter activation, the global impact of p73α on MYCN mRNA levels is inhibitory, suggesting that p73α exerts a double effect on MYCN gene expression: a positive transcriptional effect and an inhibitory post-transcriptional effect, the latter being dominant. On the other hand, p73β acts through a different mechanism, leading to MYCN repression at the transcriptional level.
We then investigated whether the transcriptional regulation of MYCN by p73 was direct using the chromatin immunoprecipitation (ChIP) approach to test the possible binding of the p73 protein to MYCN promoter. We performed ChIP analysis on LAN-1 cells that express high endogenous p73 (Figure 1). Immunoprecipitated DNA was amplified by polymerase chain reaction (PCR) using 3 pairs of primers spanning the transcription start site and proximal (1.2 kb) promoter region of the MYCN gene. We found no significant binding of the p73 protein to the MYCN promoter, while a PCR analysis of the same ChIP extracts demonstrated binding of p73 to the p75NTR promoter (Supplementary Figure S4). These results indicate that the transcriptional activation of MYCN by p73 may not be direct, although we cannot exclude that p73 interacts with MYCN gene regulatory elements located outside of the proximal promoter region.
p73α isoforms activate MYCN promoter independently of MYCN protein expression
Certain transcriptional targets induced by MYCN, in particular the MYC superfamily transcription factor MXI1, are known to repress MYCN promoter (23–25). We hypothesized that the positive effects of p73 we observed on MYCN promoter could be due to such an indirect negative feedback loop. Namely, as p73 impedes on MYCN protein levels, a resulting down-regulation of the repressive factor MXI1 could lead to increased MYCN promoter activity. To address this hypothesis, we investigated the impact of p73 on MYCN promoter activity in two MYCN non-amplified cell lines (SK-N-AS and SH-SY5Y, respectively harbouring two and three copies of the MYCN gene) that lack significant MYCN protein expression. Ectopic expression of both TA- and ΔN-p73α isoforms resulted in MYCN promoter activation in p73-negative SK-N-AS cells, while silencing of the endogenous TAp73α in SH-SY5Y cells led to diminished MYCN promoter activity (Figure 3C and D). These observations indicate that p73α isoforms lead to MYCN promoter activation independently of MYCN protein expression and of a negative feedback loop.
The p73 protein interacts with MYCN mRNA
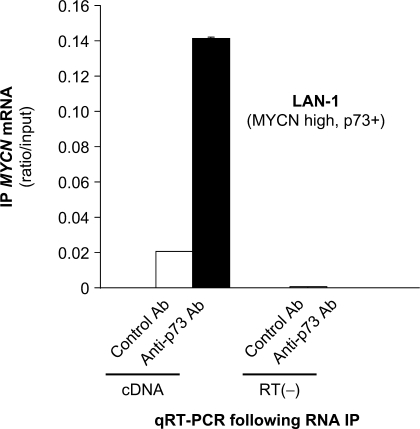
The p53 protein has been shown to interact with RNA (26). Although the exact role of these interactions is still unclear, it has been suggested that they are putatively involved in post-transcriptional gene regulation by p53. Given our observations indicating that the closely related p73α protein post-transcriptionally inhibits the MYCN gene, we investigated whether this protein could bind to MYCN transcripts. To address this question, we performed an RNA immunoprecipitation (RNA IP) analysis on LAN-1 cells that highly express both endogenous p73 protein and MYCN mRNA (Figure 1). Immunoprecipitated RNA was purified and p73-RNA interactions were evaluated by quantitative reverse-transcription polymerase chain reaction (qRT–PCR). Background MYCN mRNA could be detected in the RNA IP performed with a control antibody, but a much higher amount of MYCN transcript was recovered with a p73 antibody (Figure 4). These results indicate that the p73 protein likely interacts with MYCN mRNA and that this interaction could play a role in the inhibitory post-transcriptional regulation exerted by p73 on MYCN.
Figure 4.
The p73 protein interacts with MYCN mRNA. Binding of the p73 protein to MYCN transcript was tested by RNA immunoprecipitation (RNA IP) in LAN-1 cells. RNA samples were purified from non-precipitated cellular lysates (input) or extracts precipitated with an antibody raised against p73 (anti-p73 Ab), or a pre-immune serum (Control Ab). Immunoprecipitated MYCN transcripts were detected using real-time quantitative reverse transcription-polymerase chain reaction (qRT–PCR). cDNA: RNA subjected to reverse transcription; RT(−): samples in which reverse transcriptase was omitted from the reaction, used as negative controls. Plotted qRT–PCR values (ratios of RNA IP/input) are the means ± SEM of three replicates.
p73α impairs MYCN mRNA stability
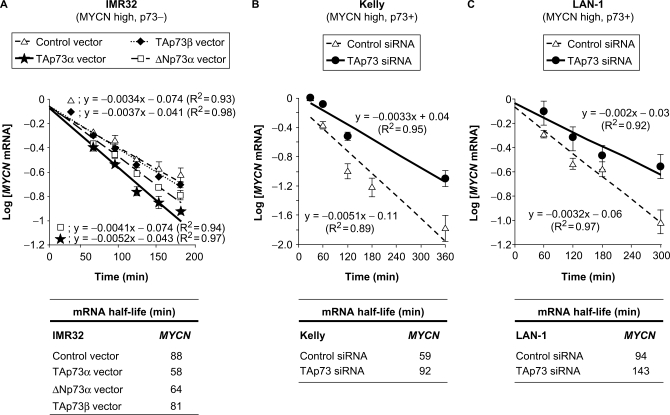
In order to further characterize the post-transcriptional inhibition of MYCN by p73, we investigated the impact of p73 on MYCN transcript stability. To assess MYCN mRNA half-life, we treated NB cells with the transcription inhibitor Actinomycin D. We then harvested cells for RNA extraction at different time points ranging from 0.5 to 6 h of treatment, and measured MYCN mRNA levels by qRT–PCR. As we observed that MYCN RNA decays in an exponential manner (Supplementary Figure S5), we plotted the logarithm of MYCN mRNA levels as a function of time. The slope of the straight lines obtained allowed us to calculate the half-life of MYCN mRNA in different experimental conditions (Figure 5). Ectopic expression of both TAp73α and ΔNp73α in IMR-32 cells led to a faster MYCN mRNA decay (35 and 25% increase, respectively), whereas TAp73β had almost no significant (<10%) effects (Figure 5A). Reciprocally, silencing of endogenous TAp73α augmented by >50% MYCN mRNA half-life in both Kelly and LAN-1 cell lines (Figure 5B and C). Our observations therefore establish that p73α inhibits MYCN transcript stability.
Figure 5.
TAp73α inhibits MYCN mRNA stability in human neuroblastoma cells. Effects of ectopic expression of TAp73α (A) or small interfering RNA (siRNA)-mediated depletion of endogenous TAp73 (B and C) on MYCN mRNA stability in neuroblastoma (NB) cell lines. NB cells were transfected with the indicated control or p73 isoforms expression vectors (A) or siRNA (B, C), then treated with Actinomycin D (5 µM) to block transcription, and collected at different time points for RNA analysis. Logarithms of MYCN mRNA levels (determined by real-time quantitative reverse transcription-polymerase chain reaction—qRT–PCR—. Plotted values are the means ± standard error of the mean—SEM—of three replicates) are displayed as a function of time. The slope of the straight lines obtained in the different experimental conditions (top panels) allowed us to calculate the half-life of MYCN mRNA in response to p73 levels engineering in the three studied cell lines (bottom panels).
The post-transcriptional inhibition exerted by p73 does not extend to the whole MYC genes family
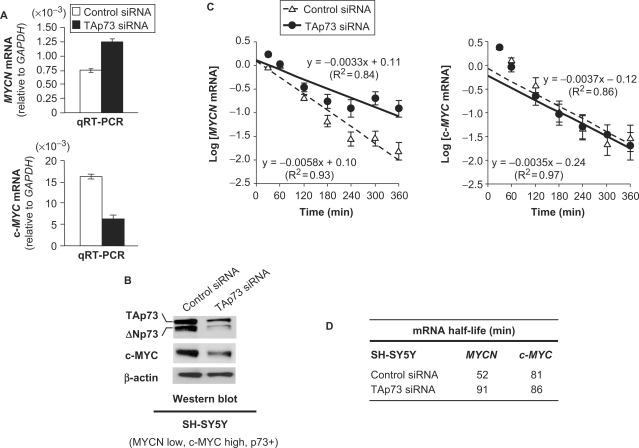
We then investigated whether the opposite transcriptional and post-transcriptional effects of p73 in NB cells was specific to MYCN. The latter belonging to the MYC proto-oncogenes family, whose sequences are very similar, we tested the impact of p73 on c-MYC, another member of this gene family. We addressed this question in the SH-SY5Y MYCN non-amplified NB cell line that exhibits low MYCN mRNA levels and expresses 20-fold more c-MYC (MYCN and c-MYC transcript levels, normalized to GAPDH, being respectively 0.76 × 10−3 and 16.3 × 10−3, see controls on Figure 6A). MYCN protein was hardly detectable in SH-SY5Y cells (Figure 1B). Nevertheless, qRT–PCR analysis showed that depletion of endogenous p73 in these cells resulted in MYCN mRNA levels increase, due to half-life augmentation (Figure 6A–D) surpassing the transcription inhibition (Figure 3D), as observed in Kelly and LAN-1 cells (Figures 2B, 3B, 5B and C). On the contrary, depletion of endogenous p73 in SH-SY5Y cells yielded an inhibition of both c-MYC mRNA and protein levels without altering c-MYC mRNA half-life (Figure 6A–D), indicating that p73 positively regulates c-MYC expression at the transcriptional level. Therefore, the negative post-transcriptional regulation exerted by p73 on MYCN mRNA is quite specific and does not operate on closely related genes.
Figure 6.
TAp73 exerts different effects on the regulation of MYCN and c-MYC expression. Effect of small interfering RNA (siRNA)-mediated depletion of endogenous TAp73 on MYCN and c-MYC mRNA (A) and c-MYC protein levels (B) in SH-SY5Y neuroblastoma (NB) cells. RNA levels were evaluated using real-time quantitative reverse transcription-polymerase chain reaction (qRT–PCR, A). Plotted qRT–PCR values are the means ± SEM of three replicates. Protein levels were monitored by western blotting (B). Transfection with TAp73 siRNA led to inhibition of both TA- and ΔN-isoforms of p73, as expected from the fact that TAp73 is known to positively regulate the expression of the ΔNp73 isoform. Control and TAp73 siRNA-transfected SH-SY5Y cells were treated with Actinomycin D (5 µM) to block transcription and collected at different time points for RNA analysis. Logarithms of MYCN and c-MYC mRNA levels (plotted values being the means ± SEM of three replicates) are displayed as a function of time. The slope of the straight lines obtained (C) allowed us to calculate the half-life of MYCN and c-MYC transcripts in response to p73 depletion in the SH SY5Y cells (D).
DISCUSSION
Our study shows that the p53 family member p73 exerts opposite transcriptional and post-transcriptional regulatory effects on MYCN gene expression in neuroblastoma cells. Indeed, although p73 isoforms TA-α and ΔN-α lead to MYCN promoter transactivation, both proteins inhibit MYCN transcript levels through a dominant post-transcriptional mechanism leading to MYCN mRNA decay, and consequently reduced protein expression. ΔNp73 isoforms, although lacking the N-terminal transactivating (TA) domain (Supplementary Figure S1), do not act systematically as dominant negatives and can activate the expression of certain genes. For example, ΔNp73α can transactivate the BTG2TIS21/PC3 gene in NB cell lines expressing wild-type p53 (27) and ΔNp73β is active in gene transactivation and growth suppression (28). The TAp73β isoform also impinges on MYCN expression, but via a distinct mechanism, as it leads to inhibition of MYCN promoter activity. These observations indicate that, unlike the N-terminal TA domain, the C-terminal region of the p73 protein contains domains which are essential for the transcriptional activation of MYCN.
As both TAp73α and ΔNp73α isoforms inhibit MYCN mRNA stability, the N-terminal TA domain of the p73 protein appears, as for the transcriptional activation of MYCN, dispensable for this post-transcriptional repressive function. On the contrary, the TAp73β isoform does not significantly alter MYCN transcript half-life, indicating that some motifs in the p73 protein C-terminal region are implicated in this post-transcriptional inhibition of MYCN. Site-directed point mutagenesis in that region will help to further specify the p73 domains involved and the molecular mechanism underlying this post-transcriptional inhibition of MYCN. This post-transcriptional inhibition exerted by p73 on MYCN does not extend to closely related genes such as c-MYC, thus indicating a relative specificity of this regulation.
The absence of physical binding between p73 and MYCN promoter, revealed by ChIP experiments, suggest that MYCN gene transactivation by p73α isoforms is indirect. It could be either due to interaction of p73 with a transcriptional repressor, thus inactivated, or mediated by a transcriptional target of p73. Alternatively, p73α isoforms may transactivate the MYCN promoter through binding to regulatory DNA elements located outside of the tested 1.2 kb proximal promoter region.
RNA IP experiments indicate that an interaction between the p73 protein and MYCN mRNA may be involved in the post-transcriptional inhibitory effects exerted by p73 on MYCN. The C-terminal domain of p73 is likely implicated in the post-transcriptional inhibition of MYCN. Indeed, the C-terminal truncated TAp73β isoform leads to transcriptional repression of MYCN but has no effect on its transcript stability. This C-terminal region of p73 contains notably a Sterile Alpha Motif (SAM) domain, some of which being known to bind to specific RNA sequences, termed SRE for SAM Response Elements (11,29,30). However, the SAM domain of p73 lacks the basic amino-acids, and is thus distinct from such SMAUG-like SAM domains. Further investigations, such as the use of engineered p73 mutants with targeted deletions, will determine the contribution of the SAM and transactivation inhibitor (TI) C-terminal domains of p73 (Supplementary Figure S1) in the post-transcriptional inhibition of MYCN. It has been reported that p53 can bind to RNA through different mechanisms (26). Three main types of p53–RNA interactions have been proposed: covalent linkage with 5.8S rRNA via aminoacid residue S389 (31), non-covalent but sequence-specific binding with CDK4 or FGF-2 mRNA (32,33), and non-covalent and non-specific binding (34). In all cases, the C-terminal domain of the p53 protein seems to be required for interaction with RNA (26). Binding of p73 to MYCN mRNA could involve mechanisms similar to those involved in p53–RNA interactions. However, it is important to note that the C-terminal domains of p53 and p73 are different.
Whatever the molecular mechanisms involved, the contradictory effects of p73 on MYCN transcription and transcript stability may reflect the necessity of a tight MYCN protein levels balance to ensure neuronal progenitor cells survival and differentiation. In the light of previous studies on the physiopathological roles of MYCN (35), disequilibrium in favour of MYCN expression abrogation would lead to neuronal cell death, while an excess of MYCN would trigger an oncogenic switch. In this respect, one report suggested that inhibition of p73 expression by MYCN could contribute to NB oncogenesis by allowing cells to escape the growth-suppressing properties of p73 (36). Given both MYCN and p73 are required for neuronal cells survival and proliferation (37–39), one can envisage, based on our observations, a coordinated action of these two proteins during neuronal cells ontogenesis. The respective contribution of p73 and MYCN proteins to neuronal cells proliferation and survival could also explain why qualitative p73 changes, rather than complete loss of p73 function, are encountered during NB oncogenesis (8,9). The dual transcriptional activation and post-transcriptional inhibition exerted by p73 on MYCN expression, revealed in our present study, might be one element in the sequence of biological events required for neuronal homoeostasis. Alterations of transcriptional and post-transcriptional effects of p73 could thus also contribute to NB oncogenesis by favouring MYCN overexpression. A recent work reported that, in HeLa cervix carcinoma cells, the p73 protein is up-regulated upon interferon-γ treatment (16). A previous study showed that, in NB cells, interferon-γ treatment leads to MYCN mRNA down-regulation, at least partly due to decreased transcript stability (17). Based on our observations, we suggest that the post-transcriptional regulation exerted by interferon-γ on MYCN in NB cells could be mediated by p73α, further arguing in favour of the possible implication of this regulation in certain physiopathological processes. In addition to the evidence provided in NB cell lines by our study, the implication of the dual regulation exerted by p73 on MYCN expression warrants further investigation in normal and cancer cells in vivo, notably using transgenic mouse models.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
ACKNOWLEDGEMENTS
We thank Dr Marc Lipinski for discussions, and Driss Chader for technical assistance. This work was supported by grants from the ‘Association pour la Recherche sur le Cancer’ (ARC, Villejuif, France) and ‘Institut National du Cancer’ (INCa, Paris, France). Emilie Horvilleur was supported by PhD fellowships from the ‘Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche’ (Paris, France) and ARC. Matthieu Bauer is supported by a PhD fellowship from the ‘Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche’. Xénia Mergui is supported by a PhD fellowship from the ‘Ligue Nationale contre le Cancer’ (Paris, France). David Goldschneider was supported by a PhD fellowship from the ‘Ligue Nationale contre le Cancer’. D. Cappellen is supported by a Junior Investigator fellowship from ARC. Funding to pay the Open Access publication charges for the article was provided by INCa. X74049.
Conflict of interest statement. None declared.
REFERENCES
- 1.Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 2.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 3.Lutz W, Leon J, Eilers M. Contributions of Myc to tumorigenesis. Biochim. Biophys. Acta. 2002;1602:61–71. doi: 10.1016/s0304-419x(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 4.Brodeur GM, Sekhon G, Goldstein MN. Chromosomal aberrations in human neuroblastomas. Cancer. 1977;40:2256–2263. doi: 10.1002/1097-0142(197711)40:5<2256::aid-cncr2820400536>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Mertens F, Johansson B, Hoglund M, Mitelman F. Chromosomal imbalance maps of malignant solid tumors: a cytogenetic survey of 3185 neoplasms. Cancer Res. 1997;57:2765–2780. [PubMed] [Google Scholar]
- 6.Fang W, Piao Z, Buyse IM, Simon D, Sheu JC, Perucho M, Huang S. Preferential loss of a polymorphic RIZ allele in human hepatocellular carcinoma. Br. J. Cancer. 2001;84:743–747. doi: 10.1054/bjoc.2000.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, Minty A, Chalon P, Lelias, J.M.Dumont X, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 8.Douc-Rasy S, Barrois M, Echeynne M, Kaghad M, Blanc E, Raguenez G, Goldschneider D, Terrier-Lacombe MJ, Hartmann O, Moll U, et al. DeltaN-p73alpha accumulates in human neuroblastic tumors. Am. J. Pathol. 2002;160:631–639. doi: 10.1016/s0002-9440(10)64883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casciano I, Mazzocco K, Boni L, Pagnan G, Banelli B, Allemanni G, Ponzoni M, Tonini GP, Romani M. Expression of DeltaNp73 is a molecular marker for adverse outcome in neuroblastoma patients. Cell Death. Differ. 2002;9:246–251. doi: 10.1038/sj.cdd.4400993. [DOI] [PubMed] [Google Scholar]
- 10.Kim CA, Bowie JU. SAM domains: uniform structure, diversity of function. Trends Biochem. Sci. 2003;28:625–628. doi: 10.1016/j.tibs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Green JB, Gardner CD, Wharton RP, Aggarwal AK. RNA recognition via the SAM domain of Smaug. Mol. Cell. 2003;11:1537–1548. doi: 10.1016/s1097-2765(03)00178-3. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, Chen X. The C-terminal sterile alpha motif and the extreme C terminus regulate the transcriptional activity of the alpha isoform of p73. J. Biol. Chem. 2005;280:20111–20119. doi: 10.1074/jbc.M413889200. [DOI] [PubMed] [Google Scholar]
- 13.Barrera FN, Poveda JA, Gonzalez-Ros JM, Neira JL. Binding of the C-terminal sterile alpha motif (SAM) domain of human p73 to lipid membranes. J. Biol. Chem. 2003;278:46878–46885. doi: 10.1074/jbc.M307846200. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa T, Takahashi M, Ozaki T, Watanabe KK, Todo S, Mizuguchi H, Hayakawa T, Nakagawara A. Autoinhibitory regulation of p73 by Delta Np73 to modulate cell survival and death through a p73-specific target element within the Delta Np73 promoter. Mol. Cell Biol. 2002;22:2575–2585. doi: 10.1128/MCB.22.8.2575-2585.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourdon JC. p53 and its isoforms in cancer. Br. J. Cancer. 2007;97:277–282. doi: 10.1038/sj.bjc.6603886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain N, Gupta S, Sudhakar C, Radha V, Swarup G. Role of p73 in regulating human caspase-1 gene transcription induced by interferon-γ and cisplatin. J. Biol. Chem. 2005;280:36664–36673. doi: 10.1074/jbc.M413261200. [DOI] [PubMed] [Google Scholar]
- 17.Wada RK, Pai DS, Huang J, Yamashiro JM, Sidell N. Interferon-gamma and retinoic acid down-regulate N-myc in neuroblastoma through complementary mechanisms of action. Cancer Lett. 1997;121:181–188. doi: 10.1016/s0304-3835(97)00351-0. [DOI] [PubMed] [Google Scholar]
- 18.De Laurenzi V, Raschella G, Barcaroli D, Annicchiarico-Petruzzelli M, Ranalli M, Catani MV, Tanno B, Costanzo A, Levrero, M.Melino G, et al. Induction of neuronal differentiation by p73 in a neuroblastoma cell line. J. Biol. Chem. 2000;275:15226–15231. doi: 10.1074/jbc.275.20.15226. [DOI] [PubMed] [Google Scholar]
- 19.Sivak LE, Tai KF, Smith RS, Dillon PA, Brodeur GM, Carroll WL. Autoregulation of the human N-myc oncogene is disrupted in amplified but not single-copy neuroblastoma cell lines. Oncogene. 1997;15:1937–1946. doi: 10.1038/sj.onc.1201363. [DOI] [PubMed] [Google Scholar]
- 20.Irwin MS, Kondo K, Marin MC, Cheng LS, Hahn WC, Kaelin W.G., Jr Chemosensitivity linked to p73 function. Cancer Cell. 2003;3:403–410. doi: 10.1016/s1535-6108(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 21.Goldschneider D, Blanc E, Raguenez G, Barrois M, Legrand A, Le Roux G, Haddada H, Benard J, Douc-Rasy S. Differential response of p53 target genes to p73 overexpression in SH-SY5Y neuroblastoma cell line. J. Cell Sci. 2004;117:293–301. doi: 10.1242/jcs.00834. [DOI] [PubMed] [Google Scholar]
- 22.Cappellen D, Schlange T, Bauer M, Maurer F, Hynes NE. Novel c-MYC target genes mediate differential effects on cell proliferation and migration. EMBO Rep. 2007;8:70–76. doi: 10.1038/sj.embor.7400849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zervos AS, Gyuris J, Brent R. Mxi1, a protein that specifically interacts with Max to bind Myc-Max recognition sites. Cell. 1993;72:223–232. doi: 10.1016/0092-8674(93)90662-a. [DOI] [PubMed] [Google Scholar]
- 24.Benson LQ, Coon MR, Krueger LM, Han GC, Sarnaik AA, Wechsler DS. Expression of MXI1, a Myc antagonist, is regulated by Sp1 and AP2. J. Biol. Chem. 1999;274:28794–28802. doi: 10.1074/jbc.274.40.28794. [DOI] [PubMed] [Google Scholar]
- 25.Dugast-Darzacq C, Pirity M, Blanck JK, Scherl A, Schreiber-Agus N. Mxi1-SRalpha: a novel Mxi1 isoform with enhanced transcriptional repression potential. Oncogene. 2004;23:8887–8899. doi: 10.1038/sj.onc.1208107. [DOI] [PubMed] [Google Scholar]
- 26.Riley KJ, Maher L.J., III p53 RNA interactions: new clues in an old mystery. RNA. 2007;13:1825–1833. doi: 10.1261/rna.673407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldschneider D, Million K, Meiller A, Haddada H, Puisieux A, Bénard J, May E, Douc-Rasy S. The neurogene BTG2TIS21/PC3 is transactivated by DeltaNp73alpha via p53 specifically in neuroblastoma cells. J. Cell Sci. 2005;118:1245–1253. doi: 10.1242/jcs.01704. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Nozell S, Xiao H, Chen X. DeltaNp73beta is active in transactivation and growth suppression. Mol. Cell Biol. 2004;24:487–501. doi: 10.1128/MCB.24.2.487-501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aviv T, Lin Z, Lau S, Rendl LM, Sicheri F, Smibert CA. The RNA-binding SAM domain of Smaug defines a new family of post-transcriptional regulators. Nat. Struct. Biol. 2003;10:614–621. doi: 10.1038/nsb956. [DOI] [PubMed] [Google Scholar]
- 30.Oberstrass FC, Lee A, Stefl R, Janis M, Chanfreau G, Allain FH. Shape-specific recognition in the structure of the Vts1p SAM domain with RNA. Nat. Struct. Mol. Biol. 2006;13:160–167. doi: 10.1038/nsmb1038. [DOI] [PubMed] [Google Scholar]
- 31.Samad A, Carroll RB. The tumor suppressor p53 is bound to RNA by a stable covalent linkage. Mol. Cell Biol. 1991;11:1598–1606. doi: 10.1128/mcb.11.3.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller SJ, Suthiphongchai T, Zambetti GP, Ewen ME. p53 binds selectively to the 5' untranslated region of cdk4, an RNA element necessary and sufficient for transforming growth factor beta- and p53-mediated translational inhibition of cdk4. Mol. Cell Biol. 2000;20:8420–8431. doi: 10.1128/mcb.20.22.8420-8431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galy B, Creancier L, Zanibellato C, Prats AC, Prats H. Tumour suppressor p53 inhibits human fibroblast growth factor 2 expression by a post-transcriptional mechanism. Oncogene. 2001;20:1669–1677. doi: 10.1038/sj.onc.1204271. [DOI] [PubMed] [Google Scholar]
- 34.Riley KJ, Cassiday LA, Kumar A, Maher L.J., III Recognition of RNA by the p53 tumor suppressor protein in the yeast three-hybrid system. RNA. 2006;12:620–630. doi: 10.1261/rna.2286706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charron J, Malynn BA, Fisher P, Stewart V, Jeannotte L, Goff SP, Robertson EJ, Alt FW. Embryonic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes Dev. 1992;6:2248–2257. doi: 10.1101/gad.6.12a.2248. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X, Wimmer K, Kuick R, Lamb BJ, Motyka S, Jasty R, Castle VP, Hanash SM. N-myc modulates expression of p73 in neuroblastoma. Neoplasia. 2002;4:432–439. doi: 10.1038/sj.neo.7900255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moens CB, Stanton BR, Parada LF, Rossant J. Defects in heart and lung development in compound heterozygotes for two different targeted mutations at the N-myc locus. Development. 1993;119:485–499. doi: 10.1242/dev.119.2.485. [DOI] [PubMed] [Google Scholar]
- 38.Pozniak CD, Radinovic S, Yang A, McKeon F, Kaplan DR, Miller FD. An anti-apoptotic role for the p53 family member, p73, during developmental neuron death. Science. 2000;289:304–306. doi: 10.1126/science.289.5477.304. [DOI] [PubMed] [Google Scholar]
- 39.Pozniak CD, Barnabe-Heider F, Rymar VV, Lee AF, Sadikot AF, Miller FD. p73 is required for survival and maintenance of CNS neurons. J. Neurosci. 2002;22:9800–9809. doi: 10.1523/JNEUROSCI.22-22-09800.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.