Abstract
DKK1 is a secreted protein that antagonizes Wnt signaling and plays essential roles in vertebrate embryogenesis including head induction, skeletal development, and limb patterning. DKK1 is also implicated in osteoporosis, arthritis, and cancer and represents a potential therapeutic target for the treatment of these diseases. DKK1 is a high affinity antagonistic ligand for LRP6, which is a Wnt coreceptor that acts together with the Frizzled serpentine receptor to initiate Wnt signal transduction. Two different models have been proposed to account for the mechanism by which DKK1 antagonizes LRP6 function. One model suggests that DKK1 binding to LRP6 disrupts Wnt-induced Frizzled-LRP6 complex formation, whereas the other model proposes that DKK1 interaction with LRP6 promotes LRP6 internalization and degradation, thereby reducing the cell surface LRP6 level. To clarify the molecular basis of DKK1 action, we examined how DKK1 affects the endogenous LRP6 in several mammalian cell lines including mouse embryonic fibroblasts. Here we show that DKK1 inhibits Wnt signaling but induces neither LRP6 down-regulation from the cell surface nor reduction of total LRP6 protein level and that DKK1 has no effect on the rate of continuous internalization of LRP6 and the half-life (about 4.7 h) of LRP6. We conclude that DKK1 inhibition of LRP6 is independent of LRP6 internalization and degradation.
Dickkopf1 (DKK1)3 is a secreted antagonist for Wnt signaling and has emerged as a key regulatory molecule for vertebrate embryonic development and human diseases (1). DKK1 was identified as a head inducer in Xenopus embryos (2), and loss-of-function studies have demonstrated its essential role in vertebrate head/anterior development (2, 3). In addition, DKK1 is involved in presomitic mesoderm and vertebrae formation (4), the formation and maintenance of the apical ectodermal ridge that directs limb patterning and growth (2–5), and many other aspects of vertebrae embryogenesis (1). Furthermore, DKK1 involvement in modulation of peak bone density (6, 7), joint remodeling (8), hair growth (9), and suppression of cancer cell proliferation (10, 11) provides the basis for developing novel therapeutic approaches for the treatment of osteoporosis, rheumatoid arthritis, baldness, and cancer.
The diverse biological roles of DKK1 rely on its ability to inhibit β-catenin signaling activated by the Wnt family of secreted signaling proteins (4, 5, 12, 13). Wnt proteins engage two distinct classes of cell surface receptors. One is a member of the Frizzled (Fz) family of seven-pass transmembrane receptors, whereas the other is the LRP6 (low density lipoprotein receptor-related protein 6) or its close relative, LRP5 (14–16). In vitro biochemical studies suggest that Wnt induces Fz-LRP6 complex formation (17, 18), which is essential for Wnt signal transduction (17, 19–22). DKK1 binds with high affinity to LRP6 and LRP5 (18, 23, 24), and this binding is essential for the ability of DKK1 to inhibit Wnt signaling (18).
DKK1 is the founding member of a conserved DKK family, which includes multiple members encoded in the genome of all vertebrates and some invertebrates such as urochordates and ascidians, but is noticeably missing from the Caenorhabditis elegans and Drosophila genomes (1). Vertebrates have four members in the DKK family (2, 25), which share two conserved cysteine rich domains, CYS1 and CYS2 (2). The N-terminal CYS1 domain is unique to DKK proteins but is not required for the ability of DKK1 to inhibit Wnt signaling, and its function is not yet defined. The C-terminal CYS2 domain is able to bind to LRP6 and LRP5 (18, 23, 24, 26, 27). How DKK1 binding to LRP6 leads to the inhibition of Wnt signaling remains not fully resolved. Two substantially different models have been proposed to account for the mechanism of Wnt signaling inhibition by DKK1 (18, 28). One model, based on the analysis of interactions between DKK1, LRP6, Wnt1, and Fz8 proteins in vitro, suggests that DKK1 prevents LRP6 interaction with the Wnt protein and disrupts Wnt-induced Fz8-LRP6 complex formation (18). Another model, based on overexpression studies in mammalian cells, suggests that DKK1 interaction with LRP6 induces LRP6 removal from the cell surface and renders cells unresponsive to Wnt proteins (28). To clarify the molecular mechanism of DKK1 action, we examined how DKK1 regulates the endogenous LRP6 in mammalian cells.
EXPERIMENTAL PROCEDURES
Cell Culture—L cells (ATCC catalog no. CRL-2648), Rat2 cells (ATCC catalog no. CRL-1764), and HEK293T cells (ATCC catalog no. CRL-11268) were obtained from ATCC. MEF cell lines were provided by B. Skarnes and B. T. MacDonald. Cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% newborn calf serum or fetal bovine serum (for MEFs) and antibiotics. DKK1 conditioned medium (CM) was obtained by transient transfection of HEK293T cells with DKK1 expression plasmid (29). Wnt3a CM and control CM were collected from L cells stably transfected with a Wnt3a-expressing plasmid (ATCC catalog no. CRL-2647) or untransfected L cells as described by Willert et al. (30). All CMs were cleared from cell debris via centrifugation for 10 min at 15,000 × g, aliquoted, and stored at -80 °C. Precipitation, immunoblotting, and the cytosolic β-catenin assay were performed as described earlier (18, 29).
Antibodies—LRP6 antibodies were raised against the entire intracellular domain of human LRP6 and were affinity-purified against the same LRP6 fragment. EGFR antibodies (Cell Signaling, antibody no. 2232), β-catenin antibodies (Sigma, antibody no. C2206), and avidin-HRP conjugate (Pierce, catalog no. 31001) were used according to manufacturers' instructions. LRP6/pcDNA3.1 plasmid was described earlier (31). EGF was obtained from Peprotech (catalog no. 315-09).
Cell Surface Protein Labeling with Biotin—Cell surface protein labeling with biotin was performed in the cold room at +4 °C using 0.5 mg/ml Sulfo-NHS-LC-Biotin (Pierce, catalog no. 21335) or cleavable Sulfo-NHS-SS-Biotin (Pierce, catalog no. 21331) (32). To remove cleavable biotin from cell surface proteins, cells were treated with glutathione solution at alkali pH (33, 34). Cell membrane proteins were extracted by 2% Triton X-100 in phosphate-buffered saline for 2 h on a rocking platform. Cell extracts were cleared by centrifugation and diluted four times with phosphate-buffered saline, and biotinylated proteins were precipitated with immobilized NeutrAvidin protein (Pierce, catalog no. 29200) or with LRP6 antibodies using GammaBind Sepharose (Amersham Biosciences, catalog no. 17-0886-01).
Measuring LRP6 Half-life—To measure LRP6 half-life, L cells were seeded on 10-cm dishes. On the second day, cell surface proteins were biotinylated, and cells were returned to the CO2 incubator and allowed to recover from the biotinylation procedure for 2 h. Following the recovery period, cells were incubated with DKK1 or control CMs for various time points as indicated, and culture dishes with cells were removed from the incubator, rinsed twice with ice-cold phosphate-buffered saline, and placed in a -80 °C freezer. Membrane proteins were extracted from all dishes at once, and biotinylated proteins were precipitated with immobilized NeutrAvidin protein. The precipitates were immunoblotted with avidin-HRP to detect changes in bulk biotinylation of cell surface proteins or with LRP6 antibodies to trace the LRP6 turnover. Immunoblotting images were acquired using LSA-3000 system (FujiFilm) and analyzed using NIH Image software.
RESULTS
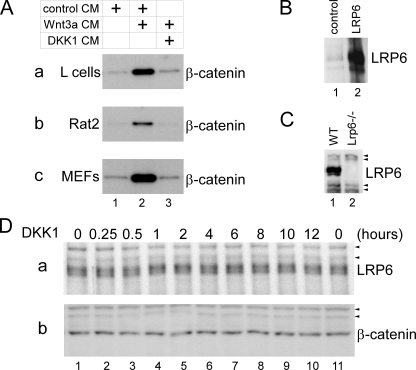
We used four different cell lines (HEK293T (human), Rat2 (rat), mouse L cells, and MEFs) to study the effect of DKK1 on the endogenous LRP6 protein. These cell lines have been commonly used in studies of Wnt signaling. Wnt3a CM robustly activated Wnt signaling in these cells as assayed by cytosolic β-catenin stabilization (Fig. 1A), and DKK1 CM efficiently blocked Wnt signaling in these cells (Fig. 1A), showing that all cellular components required for the inhibition of Wnt signaling by DKK1 are present in each of these cells. We used rabbit polyclonal antibodies (Abs) to examine the endogenous LRP6 protein. These Abs recognized two bands of molecular mass of 200 and 210 kDa on immunoblotting of the cell extract of HEK293T cells that overexpressed LRP6 and also detected the same sized bands of much lower levels in the cell extract from control-transfected HEK293T cells, which presumably represent the endogenous LRP6 in these cells (Fig. 1B). Importantly, these Abs detected two bands of the same sizes in the extract of the wild-type MEFs, but not of LRP6-/- MEFs (Fig. 1C). These results demonstrated that the Abs specifically recognize the endogenous LRP6 protein.
FIGURE 1.
DKK1 does not affect the amount of endogenous LRP6. A, DKK1 inhibits signaling by Wnt3a in L, Rat2, and MEF cells. L cells (panel a), Rat2 cells (panel b), and MEFs (panel c) were incubated for 2 h with control CM (lane 1), Wnt3a CM mixed with control CM (lane 2), or Wnt3a CM mixed with DKK1 CM (lane 3). Cytosolic fractions were obtained and analyzed by immunoblotting with β-catenin antibodies. B, LRP6 antibodies recognize LRP6 overexpressed in HEK293T cells. Total cell lysates of HEK293T transfected with control (lane 1) or LRP6-expressing plasmid (lane 2) were analyzed by immunoblotting with LRP6 antibodies. The same sized bands of much weaker intensity in the control lysate likely represent the endogenous LRP6. C, LRP6 antibodies recognize bands with a molecular mass of ∼200 and 210 kDa in the total lysate of the wild-type MEFs (lane 1) but not of Lrp6-/- MEFs (lane 2). Arrowheads indicate nonspecific bands recognized by secondary antibodies. WT, wild-type. D, DKK1 treatment of L cells does not lead to any decrease in the endogenous LRP6 protein level. L cells were treated with DKK1 CM for different lengths of time as indicated, and total cell lysates were obtained and analyzed with LRP6 (panel a) or β-catenin (panel b) antibodies. β-catenin and nonspecific bands (arrowheads) serve as loading controls.
We first studied whether DKK1 affects the total LRP6 protein level. L cells were treated with DKK1 for different lengths of time, and cell extracts from control and DKK1-treated cells were analyzed with the LRP6 Abs. We found that the amount of the endogenous LRP6 showed no appreciable changes upon DKK1 treatment, regardless of the length of the treatment (from 15 min to 12 h (Fig. 1D, panel a). And as expected, we observed no changes in the cytosolic β-catenin levels following DKK1 treatment (Fig. 1D, panel b).
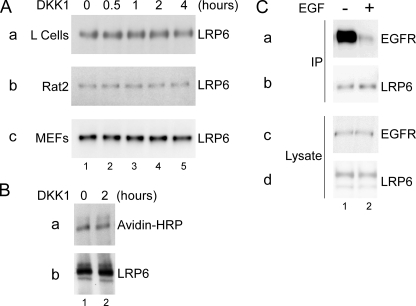
We next studied whether DKK1 affects the amount of LRP6 on the cell surface. Cells were treated with DKK1 CM for different lengths of time. After DKK1 treatment, cell surface proteins were biotinylated, extracted and precipitated with avidin-agarose beads, and immunoblotted for LRP6 protein. We found that DKK1 treatment for up to 4 h did not affect the amount of LRP6 on the cell surface in L, Rat2, and MEF cells at any time point (Fig. 2A). Similarly, DKK1 treatment had no effect on the cell surface and total LRP6 level in HEK293T cells (Fig. 2B). To confirm that the technique we employed was adequate to detect ligand-induced cell surface receptor down-regulation, we treated Rat2 cells with EGF for 15 min and found that such treatment caused a dramatic reduction of the EGFR amount on the cell surface (Fig. 2C) as previously demonstrated (35).
FIGURE 2.
DKK1 does not affect the cell surface LRP6 level. A, DKK1 does not affect the cell surface LRP6 level in L (panel a), Rat2 (panel b), and MEF (panel c) cells. After cells were treated with DKK1 CM for the indicated period of time, cell surface proteins were biotinylated, extracted and precipitated with avidin-agarose beads, and analyzed for the presence of LRP6 protein by immunoblotting. B, DKK1 does not affect the cell surface or total LRP6 level in HEK293T cells. HEK293T cells were untreated (lane 1) or treated with DKK1 CM for 2 h (lane 2), and cell surface proteins were biotinylated, extracted, and precipitated with LRP6 antibodies. The amount of LRP6 on the cell surface was determined via immunoblotting with avidin-HRP (panel a), and the total amount of LRP6 in precipitates was determined with the LRP6 antibodies (panel b). C, EGF induces rapid down-regulation of EGFR on the cell surface. Rat2 cells were untreated (lane 1) or treated with 100 ng/ml EGF for 15 min (lane 2), and cell surface proteins were biotinylated, extracted, precipitated with avidin-agarose beads, and analyzed for the presence of EGFR (panel a) or LRP6 (panel b) by immunoblotting. Cell extracts were also analyzed for the total EGFR (panel c) and LRP6 (panel d) proteins.
We further examined whether DKK1 has any effect on the rate of LRP6 internalization. We labeled surface proteins of L cells with a reversible biotinylating agent, Sulfo-NHS-SS-Biotin, and treated these labeled cells with DKK1 for different lengths of time to allow internalization of cell surface proteins including LRP6. After the treatment, biotin was removed from all proteins remaining on the cell surface (33). During DKK1 treatment, internalized cell surface proteins, including LRP6, become protected from the biotin removal procedure. These proteins were extracted and precipitated with avidin-agarose beads and analyzed by immunoblotting (Fig. 3A). We found that in control cells, a small portion of LRP6 remained biotinylated after biotin removal from proteins on the cell surface, showing that LRP6 is continuously internalized even in the absence of DKK1 (Fig. 3A, lanes 1 and 2). Importantly, the amount of internalized LRP6 did not change in the presence of DKK1 regardless of the length of the DKK1 treatment, showing that DKK1 did not affect the internalization rate of the endogenous LRP6 (Fig. 3A). At the same time, in the control experiment, we found that EGF treatment of Rat2 cells for 15 min resulted in the internalization of a significant portion of the endogenous EGFR (Fig. 3B).
FIGURE 3.
DKK1 does not affect LRP6 internalization rate. A, cell surface proteins of L cells were biotinylated with a reversible biotinylation agent, and cells were incubated with DKK1 CM for the indicated periods of time to allow the internalization of cell surface proteins including LRP6 to occur. The biotinylated proteins that remain on the surface were stripped of biotin via a reducing agent that is unable to permeabilize the plasma membrane (lanes 2–6). Internalized cell surface proteins that were protected from biotin stripping were precipitated with avidin-agarose beads and analyzed by immunoblotting with LRP6 antibodies. Lane 1, control treatment without the reducing agent. B, EGF induces rapid internalization of EGFR. Cell surface proteins of Rat2 cells were biotinylated with the same reversible biotinylation agent. The cells were incubated with or without 100 ng/ml EGF for 15 min as indicated and were stripped of biotin via the same reducing agent as above (lanes 1 and 2) or were left unstripped (lanes 3 and 4). Biotinylated proteins were precipitated with avidin-agarose beads, and the EGFR amount in precipitates (panel a) or cell lysates (panel b) was analyzed by immunoblotting with EGFR antibodies. IP, immunoprecipitation.
To study the effect of DKK1 on the turnover rate of LRP6 on the plasma membrane, we biotinylated cell surface proteins of L cells (i.e. pulse) and incubated these cells for different lengths of time with control or DKK1 CM (i.e. chase). Biotinylated proteins were extracted, precipitated with avidin-agarose beads, and immunoblotted with the LRP6 Abs. In these “pulse-chase” experiments, we found that the extent and dynamics of LRP6 turnover were similar in cells treated with control or DKK1 CM (Fig. 4, A and B), with the half-life of LRP6 ∼4.7 ± 0.7 h in both cases. We did not observe any significant changes in the biotinylation levels of major cell surface proteins even after 8 h (Fig. 4A, panel c), showing that the half-life of LRP6 is significantly shorter than these major cell surface proteins.
FIGURE 4.
DKK1 does not affect LRP6 turnover on the cell surface, and DKK1 pretreatment does not affect cellular responsiveness to Wnt3a. A, cell surface proteins of L cells were biotinylated first, and the cells were incubated for the indicated period of time with control (panel a) or DKK1 CM (panel b). Biotinylated cell surface proteins were extracted and precipitated with avidin-agarose beads, and the LRP6 amount in precipitates was determined by immunoblotting with LRP6 antibodies. Lysates obtained from cells treated with control CM were also immunoblotted with avidin-HRP to detect all biotinylated cell surface proteins (panel c). The asterisk is the position corresponding to LRP6 protein. B, graphic representation of averaged results obtained from four independent experiments as in A. The starting amount of biotinylated LRP6 at zero hour is set as 1. C, DKK1 pretreatment does not affect cell responsiveness to Wnt3a. Rat2 cells were untreated (lanes 1 and 2) or pretreated with DKK1 CM for the indicated period of time (lanes 3–6). Control or DKK1 CM was replaced (lanes 2–6) with Wnt3a CM and incubated for 2 h. After incubation, cytosolic fractions were obtained and analyzed for β-catenin protein amount by immunoblotting. Nonspecific bands (arrowheads) served as loading controls. D, DKK1 pretreatment does not sensitize Wnt inhibition by subsequent DKK1 treatment. Rat2 cells were untreated (lanes 1 and 2) or pretreated with DKK1 CM for the indicated period of time (lanes 3–6). Control or DKK1 CM was replaced with Wnt3a CM (lane 2) or with Wnt3a CM plus DKK1 CM mixed at different ratios (lanes 3–6, panels a–c) and incubated for 2 h. After incubation, cytosolic fractions were obtained and analyzed for β-catenin protein amount by immunoblotting. E, DKK1 remains active after incubation for 2 h with cells. Rat2 cells were untreated (lane 1), treated with Wnt3a CM alone (lane 2), with Wnt3a CM plus fresh DKK1 CM (lane 3), or with Wnt3a CM plus used DKK1 CM (*) that had been preincubated with other Rat2 cells for 2 h (lane 4). After a 2-hour incubation, cytosolic fractions were obtained and analyzed for β-catenin protein amount by immunoblotting.
To exclude the possibility that biotinylation altered LRP6 properties, we determined the extent of LRP6 biotinylation in our experiments and whether biotinylation of cell surface proteins affects signaling by Wnt3a and inhibition by DKK1. We found that the majority of LRP6 molecules on the cell surface became biotinylated in our experiments (Fig. 5A). We also found that Wnt3a induced the same elevation of cytosolic β-catenin at different time points in control cells and cells subjected to biotinylation (Fig. 5B, panel a). In addition, biotinylation did not affect Wnt3a induction of LRP6 phosphorylation (Fig. 5B, panel c), which reflects LRP6 activation (36). Addition of DKK1 to Wnt3a CM caused complete inhibition of β-catenin accumulation and LRP6 phosphorylation in both control and biotinylated cells, showing that biotinylation had no adversary effect on DKK1 inhibitory activity (Fig. 5B, lanes 5 and 10). Thus biotinylation did not cause changes in signaling properties of LRP6 on the cell surface.
FIGURE 5.
Biotinylation of cell surface proteins does not affect cell responsiveness to Wnt3a and DKK1. A, a majority of LRP6 molecules on the cell surface became biotinylated. Cell surface proteins of L cells were biotinylated (lanes 1, 3, and 5) or mock-treated (lanes 2, 4, and 6), cells were extracted, and extracts were cleared of biotinylated proteins via avidin-agarose beads. Cell extract before (lanes 1 and 2) and after depletion of biotinylated proteins (lanes 3 and 4) and precipitates from avidin-agarose beads (lanes 5 and 6) were immunoblotted with LRP6 Abs (panel a) or avidin-HRP (panel b). IP, immunoprecipitation. B, LRP6 remains active after biotinylation. L cells were biotinylated (lanes 1–5) or mock-treated (lanes 6–10) and then incubated for the indicated periods of time with Wnt3a (lanes 2–4 and 7–9) or with a mixture of Wnt3a plus DKK1 CM (lanes 5 and 10). Total cell proteins were analyzed by immunoblotting for β-catenin (panel a), LRP6 (panel b), LRP6 phosphorylation on serine 1490 (panel c), or biotinylated proteins (panel d).
We also examined whether DKK1 pretreatment would reduce cell responsiveness to Wnt3a, a predicted outcome if DKK1 induces LRP6 removal from the cell surface and its degradation. We pretreated Rat2 cells with DKK1 CM for different amounts of time (Fig. 4C, lanes 3–6) and then stimulated these cells with Wnt3a CM (lanes 2–6). We found that Wnt3a-induced β-catenin stabilization was not affected by prior DKK1 treatment ranging from 15 min to 2 h (Fig. 4C). We also found that the same DKK1 pretreatment did not enhance or sensitize Wnt inhibition by a subsequent addition of DKK1 at different doses (Fig. 4D). To ensure that DKK1 CM maintained its Wnt inhibitory activity during the 2-h pretreatment period, we treated Rat2 cells for 2 h with DKK1 CM and then removed the DKK1 CM, mixed it with Wnt3a CM, and applied it to naive Rat2 cells (Fig. 4E). We found that incubation of DKK1 for 2 h with cells did not affect DKK1 ability to block Wnt3a activity when compared with the fresh DKK1 CM (Fig. 4E). Together these results demonstrate that DKK1 treatment does not reduce Wnt responsiveness and are consistent with the notion that DKK1 relies on direct competition to antagonize Wnt3a via binding to LRP6 but not on LRP6 removal from the cell surface.
DISCUSSION
DKK1 is a prototypic Wnt signaling inhibitor that binds to and antagonizes the function of LRP6 (18, 23, 24). DKK1 is critical for vertebrate development and is implicated in several human diseases including cancer and osteoporosis (1) and is of significant therapeutic interests. But how DKK1 antagonizes LRP6 function has not yet been fully resolved. One popular view that has been widely cited in the literature is that DKK1 induces LRP6 internalization from the cell surface and subsequent LRP6 degradation, thereby rendering cells less or nonresponsive to Wnt signaling (28). In this study, we provide several lines of evidence demonstrating that DKK1 inhibition of Wnt signaling is independent of LRP6 internalization and degradation. Using rabbit polyclonal Abs that specifically recognize the endogenous LRP6 (Fig. 1), we examined the effect of DKK1 on LRP6 in several mammalian cell lines including HEK293T, Rat2, L, and MEF cells. Each of these cell types is responsive to Wnt activation and to DKK1 inhibition and is commonly used in Wnt signaling studies. Contrary to the prevailing view, we found that DKK1 affects none of the following: (i) the total LRP6 protein level (Fig. 1); (ii) the amount of LRP6 on the cell surface (Fig. 2); (iii) the rate of LRP6 internalization, which appears to be constitutive (Fig. 3); or (iv) the half-life of LRP6 on the cell surface (Fig. 4). Furthermore, we found that prior treatment with DKK1 has no effect on the Wnt responsiveness of the cell, further implying that the cell surface LRP6 level is not significantly reduced by DKK1 (Fig. 4).
The model that DKK1 down-regulates the LRP6 cell surface level was derived from a study of a putative DKK1 receptor, Kremen2 (Krm2), which is a transmembrane protein (28). DKK1 treatment of HEK293T cells that overexpressed both LRP6 and Krm2 induced the removal of overexpressed LRP6 from the cell surface and a significant decrease of the total overexpressed LRP6 amount (28). These results led to the suggestion that DKK1 might trigger LRP6 internalization from the cell surface via the formation of a ternary complex involving DKK1, LRP6, and Krm2 and subsequent LRP6 degradation (28). However, the role of Krm2 in DKK1-induced LRP6 “internalization” has been rather obscure, as a Krm2 mutant, which lacks the intracellular and the transmembrane domains but is tethered to the plasma membrane via a glycosylphosphatidylinositol anchor, is functionally indistinguishable from the wild-type Krm2 (28). This result suggests that the Krm2 intracellular domain is dispensable for LRP6 internalization. Recently, the same group (37) showed that Krm2 binds to LRP6 directly in the absence of DKK1 and promotes LRP6 cell surface localization and LRP6 signaling. Therefore, the role of Krm2 in the regulation of LRP6 cell surface expression appears to be complex and requires further clarification. Regardless of whether or how Krm2 regulates LRP6 in the absence or presence of DKK1, our study of the endogenous LRP6 protein provides compelling evidence that DKK1 antagonizes LRP6 function via a mechanism that is independent of LRP6 internalization and degradation. In fact, our data are fully compatible with the view that DKK1 competes directly with Wnt proteins for LRP6 binding, thereby disrupting Wnt-induced Fz-LRP6 complex formation as previously demonstrated (18). We further note that, as various models have been proposed on the regulation of LRP6 endocytosis and signaling kinetics using LRP6 overexpression, our results highlight potential critical differences between the behavior of overexpressed versus the endogenous LRP6 and underscore the importance of studying the endogenous LRP6, such as by this and other studies (21, 38, 39).
Acknowledgments
We thank members of the Xi He laboratory for suggestions and help. We also thank Drs. Yonghe Li and Guojun Bu for communications and Dr. Bryan T. MacDonald and Misha M. Semenov for discussion and comments with the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1-GM57603 (to X. H.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: DKK1, Dickkopf1; Fz, Frizzled; CM, conditioned medium; MEF, mouse embryonic fibroblast; EGF, epidermal growth factor; EGFR, EGF receptor; HRP, horseradish peroxidase; Ab, antibody.
References
- 1.Niehrs, C. (2006) Oncogene 25 7469-7481 [DOI] [PubMed] [Google Scholar]
- 2.Glinka, A., Wu, W., Delius, H., Monaghan, A. P., Blumenstock, C., and Niehrs, C. (1998) Nature 391 357-362 [DOI] [PubMed] [Google Scholar]
- 3.Mukhopadhyay, M., Shtrom, S., Rodriguez-Esteban, C., Chen, L., Tsukui, T., Gomer, L., Dorward, D. W., Glinka, A., Grinberg, A., Huang, S. P., Niehrs, C., Belmonte, J. C., and Westphal, H. (2001) Dev. Cell 1 423-434 [DOI] [PubMed] [Google Scholar]
- 4.MacDonald, B. T., Adamska, M., and Meisler, M. H. (2004) Development (Camb.) 131 2543-2552 [DOI] [PubMed] [Google Scholar]
- 5.Adamska, M., MacDonald, B. T., Sarmast, Z. H., Oliver, E. R., and Meisler, M. H. (2004) Dev. Biol. 272 134-144 [DOI] [PubMed] [Google Scholar]
- 6.Morvan, F., Boulukos, K., Clement-Lacroix, P., Roman Roman, S., Suc-Royer, I., Vayssiere, B., Ammann, P., Martin, P., Pinho, S., Pognonec, P., Mollat, P., Niehrs, C., Baron, R., and Rawadi, G. (2006) J. Bone Miner. Res. 21 934-945 [DOI] [PubMed] [Google Scholar]
- 7.MacDonald, B. T., Joiner, D. M., Oyserman, S. M., Sharma, P., Goldstein, S. A., He, X., and Hauschka, P. V. (2007) Bone 41 331-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diarra, D., Stolina, M., Polzer, K., Zwerina, J., Ominsky, M. S., Dwyer, D., Korb, A., Smolen, J., Hoffmann, M., Scheinecker, C., van der Heide, D., Landewe, R., Lacey, D., Richards, W. G., and Schett, G. (2007) Nat. Med. 13 156-163 [DOI] [PubMed] [Google Scholar]
- 9.Andl, T., Reddy, S. T., Gaddapara, T., and Millar, S. E. (2002) Dev. Cell 2 643-653 [DOI] [PubMed] [Google Scholar]
- 10.Aguilera, O., Fraga, M. F., Ballestar, E., Paz, M. F., Herranz, M., Espada, J., Garcia, J. M., Munoz, A., Esteller, M., and Gonzalez-Sancho, J. M. (2006) Oncogene 25 4116-4121 [DOI] [PubMed] [Google Scholar]
- 11.Mikheev, A. M., Mikheeva, S. A., Liu, B., Cohen, P., and Zarbl, H. (2004) Carcinogenesis 25 47-59 [DOI] [PubMed] [Google Scholar]
- 12.Kelly, O. G., Pinson, K. I., and Skarnes, W. C. (2004) Development (Camb.) 131 2803-2815 [DOI] [PubMed] [Google Scholar]
- 13.Carter, M., Chen, X., Slowinska, B., Minnerath, S., Glickstein, S., Shi, L., Campagne, F., Weinstein, H., and Ross, M. E. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 12843-12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon, M. D., and Nusse, R. (2006) J. Biol. Chem. 281 22429-22433 [DOI] [PubMed] [Google Scholar]
- 15.Nusse, R. (2005) Cell Res. 15 28-32 [DOI] [PubMed] [Google Scholar]
- 16.He, X., Semenov, M., Tamai, K., and Zeng, X. (2004) Development (Camb.) 131 1663-1677 [DOI] [PubMed] [Google Scholar]
- 17.Tamai, K., Semenov, M., Kato, Y., Spokony, R., Liu, C., Katsuyama, Y., Hess, F., Saint-Jeannet, J. P., and He, X. (2000) Nature 407 530-535 [DOI] [PubMed] [Google Scholar]
- 18.Semenov, M. V., Tamai, K., Brott, B. K., Kuhl, M., Sokol, S., and He, X. (2001) Curr. Biol. 11 951-961 [DOI] [PubMed] [Google Scholar]
- 19.Liu, G., Bafico, A., and Aaronson, S. A. (2005) Mol. Cell. Biol. 25 3475-3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cong, F., Schweizer, L., and Varmus, H. (2004) Development (Camb.) 131 5103-5115 [DOI] [PubMed] [Google Scholar]
- 21.Zeng, X., Huang, H., Tamai, K., Zhang, X., Harada, Y., Yokota, C., Almeida, K., Wang, J., Doble, B., Woodgett, J., Wynshaw-Boris, A., Hsieh, J. C., and He, X. (2008) Development (Camb.) 35 367-375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tolwinski, N. S., Wehrli, M., Rives, A., Erdeniz, N., DiNardo, S., and Wieschaus, E. (2003) Dev. Cell 4 407-418 [DOI] [PubMed] [Google Scholar]
- 23.Bafico, A., Liu, G., Yaniv, A., Gazit, A., and Aaronson, S. A. (2001) Nat. Cell Biol. 3 683-686 [DOI] [PubMed] [Google Scholar]
- 24.Mao, B., Wu, W., Li, Y., Hoppe, D., Stannek, P., Glinka, A., and Niehrs, C. (2001) Nature 411 321-325 [DOI] [PubMed] [Google Scholar]
- 25.Krupnik, V. E., Sharp, J. D., Jiang, C., Robison, K., Chickering, T. W., Amaravadi, L., Brown, D. E., Guyot, D., Mays, G., Leiby, K., Chang, B., Duong, T., Goodearl, A. D., Gearing, D. P., Sokol, S. Y., and McCarthy, S. A. (1999) Gene 238 301-313 [DOI] [PubMed] [Google Scholar]
- 26.Brott, B. K., and Sokol, S. Y. (2002) Mol. Cell. Biol. 22 6100-6110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, L., Mao, J., Sun, L., Liu, W., and Wu, D. (2002) J. Biol. Chem. 277 5977-5981 [DOI] [PubMed] [Google Scholar]
- 28.Mao, B., Wu, W., Davidson, G., Marhold, J., Li, M., Mechler, B. M., Delius, H., Hoppe, D., Stannek, P., Walter, C., Glinka, A., and Niehrs, C. (2002) Nature 417 664-667 [DOI] [PubMed] [Google Scholar]
- 29.Semenov, M., Tamai, K., and He, X. (2005) J. Biol. Chem. 280 26770-26775 [DOI] [PubMed] [Google Scholar]
- 30.Willert, K., Brown, J. D., Danenberg, E., Duncan, A. W., Weissman, I. L., Reya, T., Yates, J. R., III, and Nusse, R. (2003) Nature 423 448-452 [DOI] [PubMed] [Google Scholar]
- 31.Brown, S. D., Twells, R. C., Hey, P. J., Cox, R. D., Levy, E. R., Soderman, A. R., Metzker, M. L., Caskey, C. T., Todd, J. A., and Hess, J. F. (1998) Biochem. Biophys. Res. Commun. 248 879-888 [DOI] [PubMed] [Google Scholar]
- 32.Semenov, M. V., and He, X. (2006) J. Biol. Chem. 281 38276-38284 [DOI] [PubMed] [Google Scholar]
- 33.Graeve, L., Drickamer, K., and Rodriguez-Boulan, E. (1989) J. Cell Biol. 109 2809-2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le, T. L., Yap, A. S., and Stow, J. L. (1999) J. Cell Biol. 146 219-232 [PMC free article] [PubMed] [Google Scholar]
- 35.Carpenter, G. (1987) Annu. Rev. Biochem. 56 881-914 [DOI] [PubMed] [Google Scholar]
- 36.Tamai, K., Zeng, X., Liu, C., Zhang, X., Harada, Y., Chang, Z., and He, X. (2004) Mol. Cell 13 149-156 [DOI] [PubMed] [Google Scholar]
- 37.Hassler, C., Cruciat, C. M., Huang, Y. L., Kuriyama, S., Mayor, R., and Niehrs, C. (2007) Development (Camb.) 134 4255-4263 [DOI] [PubMed] [Google Scholar]
- 38.Wei, Q., Yokota, C., Semenov, M. V., Doble, B., Woodgett, J., and He, X. (2007) J. Biol. Chem. 282 15903-15911 [DOI] [PubMed] [Google Scholar]
- 39.Khan, Z., Vijayakumar, S., de la Torre, T. V., Rotolo, S., and Bafico, A. (2007) Mol. Cell. Biol. 27 7291-7301 [DOI] [PMC free article] [PubMed] [Google Scholar]