Abstract
Some pathogens are capable of suppressing the melanization response of host insects, but the virulence factors responsible are largely unknown. The insect pathogen Microplitis demolitor bracovirus encodes the Egf family of small serine proteinase inhibitors. One family member, Egf1.0, was recently shown to suppress melanization of hemolymph in Manduca sexta in part by inhibiting the enzymatic activity of prophenoloxidase activating proteinase 3 (PAP3). However, other experiments suggested this viral protein suppresses melanization by more than one mechanism. Here we report that Egf1.0 inhibited the amidolytic activity of PAP1 and dose-dependently blocked processing of pro-PAP1 and pro-PAP3. Consistent with its PAP inhibitory activity, Egf1.0 also prevented processing of pro-phenoloxidase, serine proteinase homolog (SPH) 1, and SPH2. Isolation of Egf1.0-protein complexes from plasma indicated that Egf1.0 binds PAPs through its C-terminal repeat domain. Egf1.0 also potentially interacts with SPH2 and two other proteins, ferritin and gloverin, not previously associated with the phenoloxidase cascade. Overall, our results indicate that Egf1.0 is a dual activity PAP inhibitor that strongly suppresses the insect melanization response.
Melanization of hemolymph is a conserved humoral immune response that is elicited in most if not all arthropods by wounding and infection. Regulation of the melanization response is controlled by one or more cascades comprised of phenoloxidases (POs)2 and other, mostly unknown, serine proteinases (1, 2). Biochemical studies in the lepidopteran Manduca sexta have identified one initiation serine proteinase (hemolymph protein 14 (HP14)) that autoactivates in the presence of microbial elicitors (3, 4), a downstream proteinase (HP21) activated by HP14, and three terminal PAPs (PAP1, 2, 3) that cleave pro-PO at its Arg-Phe reactive site bond to form PO. Activated PO then catalyzes the formation of reactive intermediates and melanin that accumulate around pathogens, encapsulated parasites, and wound sites (4–8). HP21 was recently shown to process pro-PAP2 and pro-PAP3 (9, 10). Full activation of PO by PAPs in M. sexta also requires processing of catalytically inactive SPH precursors (8, 11), whereas negative regulation of the PO cascade involves at least four serpins that inhibit HP21, PAPs, or other HPs (2). Biochemical studies in the silkmoth Bombyx mori and beetle Holotrichia diomphalia have identified other PAPs (named PPAF (pro-phenoloxidase activating factor) and PPAE (pro-phenoloxidase activating enzyme)) (12, 13), whereas genetic screens in Drosophila melanogaster and the mosquito Anopheles gambiae have identified additional serine proteinases with mostly unknown functions in the melanization process (14–17).
Several pathogens and parasites of insects have evolved counterstrategies for suppressing melanization and other host immune defenses (18–20). Relatively little is known, however, about the virulence factors responsible or the host molecules with which they interact. Thousands of parasitoid wasps in the families Braconidae and Ichneumonidae carry polydnaviruses that females inject into host insects when ovipositing (21, 22). Polydnaviruses infect multiple tissues of the host and in the absence of replication express several gene products that prevent the host's immune system from killing the wasp's offspring (19–21). The braconid Microplitis demolitor parasitizes several species of Lepidoptera and carries M. demolitor bracovirus. M. demolitor bracovirus encodes multiple virulence genes including the Egf family that consists of small serine proteinase inhibitor (smapin) homologs (23–25). One family member, Egf1.0, suppresses melanization of hemolymph in M. sexta and other insect species, whereas a second, structurally similar member, Egf0.4, exhibits no anti-melanization activity (26).
Most known smapins are small proteins that consist of a cysteine-rich, trypsin inhibitor-like domain (CD) and are thought to function as competitive inhibitors of specific target enzymes (27). Egf0.4 shares this structure, whereas Egf1.0 is a larger, compound protein that consists of an N-terminal CD and a C-terminal repeat domain (RD). M. sexta PAPs hydrolyze IEAR-p-nitroanilide (IEARpNA) and other artificial substrates with a P1 arginine. Wild-type Egf1.0 competitively inhibits PAP3 amidolytic activity and is cleaved by PAP3 at its predicted reactive site (LCYR↓FQQF) in the CD, whereas Egf0.4 exhibits no PAP3 inhibitory activity even though its reactive site (FCFK↓FTTV) is similar. The replacement mutant Egf1.0R51A, whose P1 arginine is replaced by an alanine, and the deletion mutant Egf1.0ΔCD that consists of only the RD both have no effect on PAP3 amidolytic activity. Each, however, retains the ability to inhibit melanization of M. sexta plasma. Reciprocally, Egf1.0ΔRD, which consists of only the CD, retains some anti-amidolytic activity toward PAP3 but lacks anti-melanization activity in plasma (26). In addition, neither wild-type Egf1.0 nor any Egf1.0 mutants have any inhibitory effect on melanization once PO is activated (26).
Taken together, these data indicate that Egf1.0 disables hemolymph melanization in M. sexta in part by competitively inhibiting the enzymatic activity of PAP3. However, the ability of Egf1.0R51A and Egf1.0ΔCD to inhibit melanization but not the amidolytic activity of PAP3 suggests that Egf1.0 disrupts the melanization process by more than one mechanism. Here we report that Egf1.0 inhibits the catalytic activity of PAP1. Our results also reveal that Egf1.0 inhibits processing of pro-PAP1 and pro-PAP3.
EXPERIMENTAL PROCEDURES
Insects and Hemolymph Collection—M. sexta eggs were purchased from Carolina Biological Supplies, and the larvae were reared on an artificial diet as previously described (26). Hemolymph used in the study was prepared by first injecting day 3, fourth instar M. sexta hemolymph with heat-killed Micrococcus luteus (1.5 × 107 colony-forming units/ml), which up-regulates expression of PO cascade components (4, 7, 11). Hemolymph was then collected 24 h later on ice and mixed with an equal volume of anticoagulant (4 mm NaCl, 40 mm KCl, 0.1% polyvinylpyrrolidone, 1.9 mm Pipes, 4.8 mm citric acid monohydrate, 13.6 mm sodium citrate, 5% sucrose, pH 6.8). The hemolymph samples were then stored at –80 °C for later use in assays.
Recombinant and Purified Proteins—Recombinant Egf1.0, Egf0.4, Egf1.0R51A, Egf1.0ΔRD, and Egf1.0ΔCD, each containing His tags, were expressed in Escherichia coli and purified as described previously (26). PAP1, pro-PO, SPH1, and SPH2 were purified from M. sexta hemolymph (28–30). Note that purified PAP1 is processed (i.e. preactivated) and, thus, fully functional to hydrolyze IEARpNA or process pro-PO. The concentration of each protein was determined using the Coomassie Plus kit (Pierce), and stock solutions were adjusted to 0.1 mg/ml.
SDS-PAGE and Immunoblotting—Proteins were analyzed by SDS-PAGE and visualized by silver staining or immunoblotting. Proteins were mixed with 5× sample buffer, boiled for 3 min, and then loaded on 7.5 or 4–20% precast SDS-PAGE gels (Lonza). For immunoblotting, proteins were transferred to polyvinylidene difluoride membrane (Immobilon-P, Millipore) after electrophoresis. Membranes were blocked with 2% nonfat milk at room temperature for 1 h and incubated with rabbit antisera to Egf1.0, Egf0.4, pro-PO, PAP1–3, SPH1 or SPH2, HP14, HP21, or serpin 3 (SPN3) (1:100,000) at 4 °C overnight (4, 6, 8, 10, 11, 33). Primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies (1: 50,000, Jackson ImmunoResearch) and visualized by ECL Advance kit (GE Healthcare).
Affinity Purification of Egf1.0-Plasma Protein Complexes—Four hundred μl of freshly collected M. sexta hemolymph from M. luteus-injected larvae was mixed with 400 μl of 2 × Ni-NTA binding buffer (25 mm Tris-HCl, pH 8.0, 100 mm NaCl, 10% glycerol) containing phenylthiourea at a final concentration of 0.001%. After centrifugation at 14,000 × g at 4 °C for 10 min, the supernatant was incubated with 50 μl of recombinant Egf1.0 (0.1 mg/ml) in Ni-NTA binding buffer at 4 °C for 90 min. Hemolymph samples were loaded onto Ni-NTA spin columns (bed volume 100 μl, Qiagen) and spun at 3000 × g for 2 min followed by reloading and spinning three additional times. The columns were washed with 800 μl of Ni-NTA binding buffer 3× before eluting with 160 μl of elution buffer (25 mm Tris-HCl, pH 8.0, 100 mm NaCl, 10% glycerol, 300 mm imidazole). Eluted proteins were then analyzed by SDS-PAGE and immunoblotting as described above.
Mass Spectrometry Analyses—To identify other proteins associated with Egf1.0, Ni-NTA column eluates were subjected to SDS-PAGE and modified Blum silver staining (31). Bands of interest were excised, reduced with Tris[2-carboxyethyl]phosphine, alkylated with iodoacetamide, and subjected to in-gel digestion with modified trypsin (Pierce) (32). Tryptic peptide pools were evaporated to near dryness and desalted on C18 Ziptips (Millipore). Samples were then sent to the University of Nevada Reno Proteomics Center where they were eluted with 70% acetonitrile, 0.2% formic acid, overlaid with 0.5 μlof5 mg/ml MALDI matrix (α-cyano-4 hydroxycinnamic acid, 10 mm ammonium phosphate), and analyzed using an ABI 4700 Proteomics Analyzer MALDI TOF/TOF mass spectrometer (Applied Biosystems, CA) and 4000 Series Explorer software Version 3.6. Spectra were calibrated with a six-peptide calibration standard mixture. The filtered data were searched December, 2007 by Mascot Version 1.9.05 (Matrix Science) using the NCBI nr data base (NCBI 20070908). Searches were performed without restriction to protein species, Mr, or pI and with variable oxidation of methionine residues and carbamidomethylation of cysteines.
Pro-PO Activation and Phenoloxidase Activity Assays—Sixty μl of hemolymph was mixed with 10 μl of Egf proteins (0.1 mg/ml) and 10 μl of insoluble β-1,3 glucan (curdlan) (10 mg/ml) that activates the PO cascade (7). Samples were placed on a rotary mixer at room temperature for 10 min. After spin-down of the curdlan, 20 μl of the supernatant was added to 100 μl of 2 mm dopamine followed by A485 measurement on a plate reader (FLUOstar Galaxy, BMG Labtech) for 20 min. Treatments were replicated three times and analyzed by one-way analysis of variance and Dunnett's multiple comparison procedure using JMP 5.0 software (SAS Institute). Activation of pro-PO in vitro was performed as outlined (11). Briefly, purified pro-PO (1 μl, 20 ng/μl) was combined with PAP1 (1 μl, 5 ng/μl) and SPH1/2 (1 μl, 20 ng/μl) and incubated in 20 mm Tris-HCl, pH 7.6, on ice with or without Egf proteins (1 μl, 10 ng/μl) for 40 min. After incubation, the reactions were subjected to PO activity assays or SDS-PAGE electrophoresis and immunoblotting as described above.
Amidase Assays—As previously noted, IEARpNA is an artificial nitroanilide substrate used to measure PAP activity. Assays were conducted by incubating PAP1 (4 μl, 5 ng/μl) with or without Egf proteins (2 μl, 0.1 mg/ml) in 20 μl of 0.1 m Tris-HCl, 0.1 m NaCl, 5 mm CaCl2, pH 7.8, at room temperature for 10 min. One hundred μl of 100 μm IEARpNA was then added, and reactions were monitored at A405 on a plate reader for 20 min. One unit of activity is defined as ΔA405/min = 0.001 (5–7). Assays were replicated three times and then analyzed as described for PO activity assays.
RESULTS
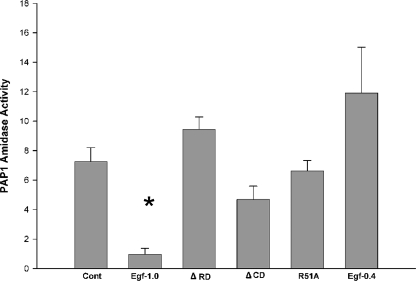
Egf1.0 Inhibits the Amidolytic Activity of PAP1—As previously noted, PAPs purified from M. sexta hemolymph are already activated and, thus, fully functional to hydrolyze synthetic substrates like IEARpNA. Previous results indicated that Egf1.0 dose-dependently inhibits the hydrolytic and pro-PO processing activity of PAP3 (26). Additional assays also confirmed that PAP3 specifically cleaves Egf1.0 at its reactive Arg(P1)-Phe(P1′) bond (26), indicating that Egf1.0 functions as a competitive inhibitor of PAP3. To ask whether Egf proteins inhibit the activity of other PAPs from M. sexta, we added recombinant Egf1.0 or Egf0.4 to purified PAP1 and measured hydrolysis of IEARpNA. These results indicated that Egf1.0 strongly inhibited the amidolytic activity of PAP1, but Egf0.4 did not (Fig. 1). As found for PAP3 (26), the alanine replacement mutant Egf10R51A had no inhibitory activity toward PAP1, indicating the reactive site arginine in the CD was essential for inhibitory activity (Fig. 1). Unlike prior results with PAP3, however, the deletion mutant Egf1.0ΔRD had no inhibitory activity against PAP1, whereas Egf1.0ΔCD reduced PAP1 amidolytic activity to levels intermediate between wild-type Egf1.0 and the negative control (Fig. 1). Taken together these results indicated that the reactive site in the CD was essential for inhibition of PAP1 but also suggested that the RD is required for interaction with this enzyme.
FIGURE 1.
Egf1.0 inhibits PAP1 amidolytic activity. Recombinant Egf1.0, Egf1.0ΔRD, Egf1.0ΔCD, Egf1.0R51A, or Egf0.4 (4 pmol) was added to purified PAP-1 (0.4 pmol) plus substrate for 10 min. PAP1 plus substrate alone served as the control (Cont). The asterisk indicates treatment with significantly lower amidase activity compared with the control (F5, 17 = 20.2; p < 0.001; followed by Dunnett's multiple comparison procedure).
Egf1.0 Inhibits Activation of Pro-PAP1 and Pro-PAP3—All known PAPs consist of one or two N-terminal clip domains linked by an intrachain disulfide bond to the C-terminal catalytic domain (5–7, 13). Activation of pro-PAPs occurs by cleavage between the clip and catalytic domains that form separate light (clip) and heavy (catalytic) chain bands under reducing conditions. Inactive, pro-PAP1 and pro-PAP3 run as ∼50-kDa bands on SDS-PAGE gels, the heavy (catalytic) chains run as ∼31- and ∼37-kDa bands, respectively, and the light (clip domain) chains run as ∼21-kDa bands (5–7, 9). In M. sexta hemolymph, processed (catalytically active) PAP1 and PAP3 rapidly form complexes with serpins of which SPN3 is the most important (33). Thus, when hemolymph is analyzed by SDS-PAGE and Western blotting, pro-PAP1 and pro-PAP3 run as ∼50-kDa bands, whereas the catalytic domains of processed PAP1 (∼31 kDa) and PAP3 (∼37 kDa) remain covalently attached to SPN3 (∼52 kDa), which results in the presence of ∼90-kDa bands. The clip domains of PAPs in contrast are released due to reduction of the disulfide bond and run as ∼21-kDa bands.
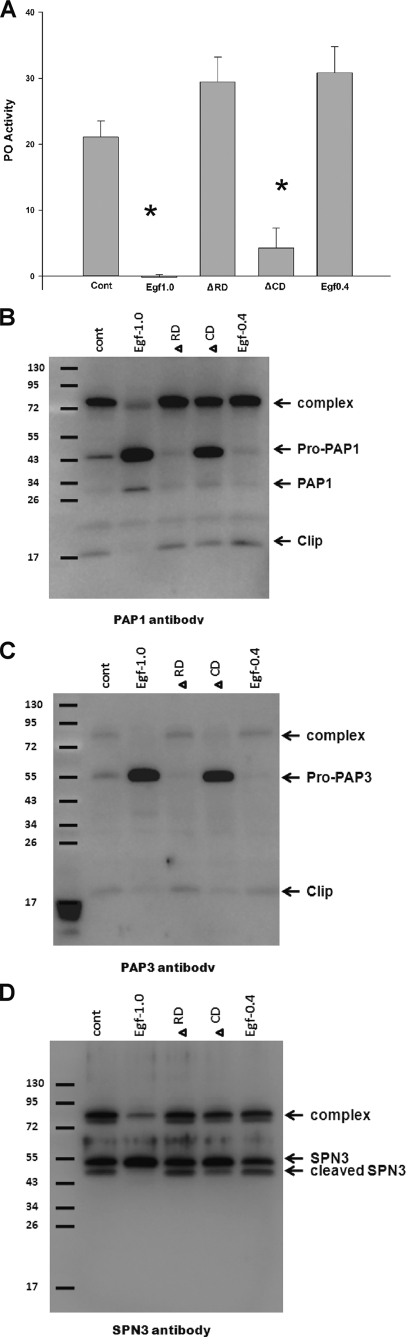
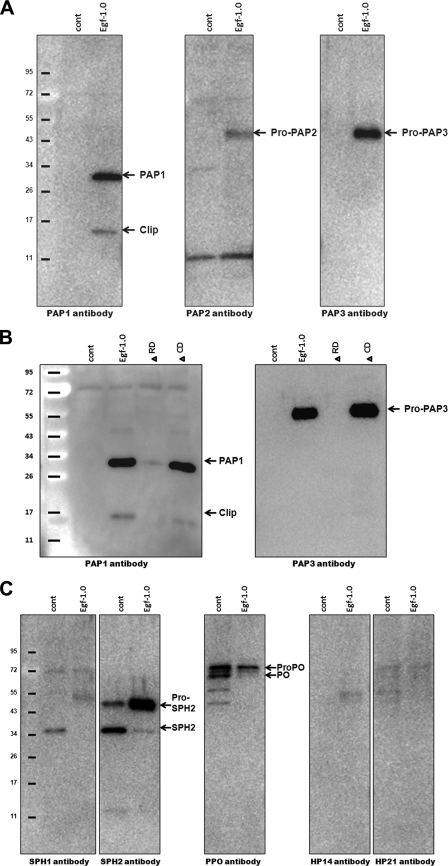
The ability of Egf1.0 to inhibit the enzymatic activity of purified PAP1 and PAP3 indicates that Egf1.0 blocks the PO cascade in part by competitively inhibiting already activated PAPs via its reactive site bond in the CD. As noted above (see the Introduction); however, the ability of Egf1.0ΔCD to inhibit melanization if added to hemolymph before activation of the PO cascade (26) strongly suggests that Egf1.0 is also able to inhibit the PO cascade by another mechanism. Given evidence that the RD of Egf1.0 is important for interaction with PAPs, we tested the hypothesis that that Egf1.0 inhibits melanization by blocking pro-PAP processing that rapidly occurs when the PO cascade is activated by immune challenge. Using the well known elicitor curdlan, PO activity assays indicated that wild-type Egf1.0 and Egf1.0ΔCD significantly reduced PO activity compared with the positive control, whereas Egf1.0ΔRD and Egf0.4 did not (Fig. 2A). Immunoblot analysis of the control, Egf1.0ΔRD, and Egf0.4 samples using PAP1, PAP3, and SPN3 antibodies confirmed the formation of ∼90-kDa PAP-serpin complexes that, as noted above, normally form after activation of the PO cascade and processing of pro-PAPs (Fig. 2, B–D). The SPN3 antibody also detected a protein of slightly lower molecular mass than SPN3 (∼57 kDa) that represents the cleaved serpin minus a 5.3-kDa fragment generated by an unknown proteinase(s) (Fig. 2D) (33). In contrast, our PAP1 and PAP3 antibodies predominantly detected ∼50-kDa bands corresponding to pro-PAPs in samples containing wild-type Egf1.0 (Fig. 2, B and C). Likewise, our SPN3 antibody primarily detected uncleaved SPN3 (Fig. 2D). For samples containing Egf1.0ΔCD, the PAP1 antibody detected bands of similar intensity corresponding to the PAP1-serpin complex and pro-PAP1 (Fig. 2B), whereas the PAP3 antibody detected a band of strong intensity for pro-PAP3 and a very weak band corresponding to the PAP3-serpin complex (Fig. 2C). Unlike the PAP1 antibody, however, the PAP3 antibody does not strongly recognize PAP3-serpin complexes. Thus, the disparity in band intensity reflected properties of the antibody rather than the abundance of pro-PAP3 and the PAP3-serpin complex.
FIGURE 2.
Egf1.0 inhibits processing of pro-PAP1 and pro-PAP3 in plasma. Egf1.0 Egf1.0ΔRD, Egf1.0ΔCD, or Egf0.4 (20 pmol) was added to plasma followed 10 min later by the addition of curdlan. Plasma plus curdlan alone served as the control (Cont). A, inhibition of PO activity. Asterisks indicate treatments with significantly lower PO activity compared with the control (F4,29 = 135.3; p < 0.001). After the addition of sample buffer, samples were subjected to SDS-PAGE under reducing conditions and immunoblotting using PAP1 (B) PAP3 (C), or SPN3 (D) antibodies. Sizes of the molecular mass markers are indicated to the left of each blot. Note that the affinity of the PAP1 and serpin3 antibodies for PAP-serpin complexes is stronger than that of the PAP3 antibody that recognizes PAP3-serpin complexes more weakly.
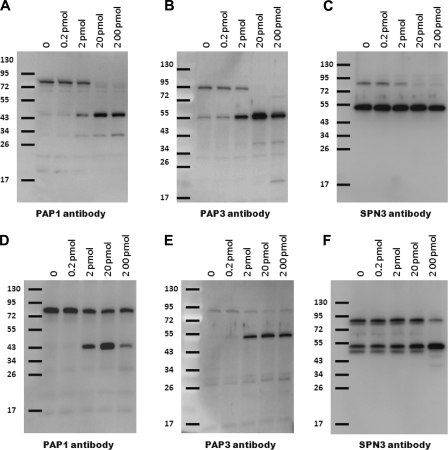
These results suggested that wild-type Egf1.0 inhibits processing of pro-PAP1 and pro-PAP3 and that this activity is partially retained by the mutant Egf1.0ΔCD. To explore this further, we added curdlan to plasma in the presence of increasing amounts of Egf1.0 or Egf1.0ΔCD (Fig. 3). These experiments indicated that wild-type Egf1.0 dose-dependently blocked processing of both pro-PAP1 and pro-PAP3, resulting in complete inhibition of PAP-serpin complex formation (Fig. 3, A–C). In contrast, Egf1.0ΔCD did not fully inhibit processing of pro-PAP1 and pro-PAP3 or PAP-serpin complex formation at any concentration tested (Fig. 3, D–F).
FIGURE 3.
Egf1.0 and Egf10ΔCD dose-dependently inhibit processing of pro-PAP1 and pro-PAP3. Increasing amounts (0–200 pmol) of Egf1.0 (A–C) or Egf1.0ΔCD (D–F) were added to plasma followed 10 min later by the addition of curdlan. Samples were then subjected to immunoblot analysis using PAP1 (A and D), PAP3 (B and E), or SPN3 (C and F) antibodies. Sizes of the molecular mass markers are indicated to the left of each blot.
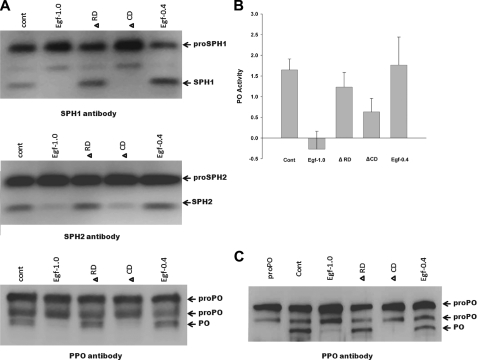
Egf1.0 Inhibits Activation of SPHs and PO—Pro-PO forms a protein complex with PAPs, SPHs, and pattern recognition proteins that is likely important for processing and localized deposition of melanin during an immune response (11). In addition, processing of pro-PO into fully active PO by PAPs requires processing of pro-SPHs into active SPH cofactors (8). We examined whether Egf proteins inhibit SPH and pro-PO processing by adding curdlan to plasma samples and immunoblotting using SPH1, SPH2, and PO antibodies. Based on antibody reactivity and size, we detected processing of pro-SPH1, pro-SPH2, and pro-PO in control samples and samples containing Egf1.0ΔRD and Egf0.4 (Fig. 4A). In contrast, little or no processing of pro-SPH1, pro-SPH2 and pro-PO occurred in samples containing Egf1.0 and Egf1.0ΔCD (Fig. 4A). To assess whether this outcome depended upon the presence of any other factors besides PAPs, SPHs, and PO, we repeated these experiments in vitro using only purified components. No PO activity was detected in samples containing pro-PO alone (data not presented). PO activity significantly increased in our positive control upon the addition of purified PAP1 and SPH1/2 (Fig. 4B). Immunoblotting confirmed this increase in PO activity correlated with processing of pro-PO to PO (Fig. 4C). The presence of wild-type Egf1.0, however, fully inhibited PO activity and pro-PO processing when PAP1 and SPH1/2 were added. Egf1.0ΔCD also reduced PO activity and pro-PO processing, but Egf1.0ΔRD and Egf0.4 did not (Fig. 4, B and C).
FIGURE 4.
Egf1.0 and Egf1.0ΔCD inhibit processing of pro-PO, pro-SPH1, and pro-SPH2. A, plasma plus curdlan only served as the positive control (cont). Egf1.0, Egf1.0ΔRD, Egf1.0ΔCD, or Egf0.4 (20 pmol) was added to plasma followed 10 min later by the addition of curdlan. After 10 min reactions were stopped by adding SDS-PAGE sample buffer and boiling for 3 min. The mixtures were then subjected to SDS-PAGE under reducing conditions and immunoblotting using SPH1, SPH2, and PO antibodies. B, inhibition of PO activity generation. Recombinant Egf1.0, Egf1.0ΔRD, Egf1.0ΔCD, or Egf0.4 (0.4 pmol) was combined with purified pro-PO (0.25 pmol) for 10 min followed by the addition of purified PAP1, SPH1, and SPH2. Samples containing pro-PO alone served as the negative control (pro-PO), whereas samples containing pro-PO plus PAP1, SPH1, and SPH2 served as the positive control (Cont). C, immunoblot of the same samples from B using the PO antibody.
Egf1.0 Binds PAP1 and PAP3 through the RD—We assessed whether Egf1.0 binds PAPs or other known proteins in the PO cascade by diluting plasma from M. luteus-challenged M. sexta in Ni-NTA binding buffer (1:1) with or without recombinant Egf1.0. We then captured Egf1.0 through its C-terminal His tag and potential interacting proteins by adding the samples to Ni-NTA spin columns followed by SDS-PAGE and immunoblot analysis of column eluates. Bands corresponding to the catalytic and clip domains of PAP1 and pro-PAP3 were detected in bead eluates of samples with Egf1.0 but not in control samples without Egf1.0 (Fig. 5A). PAP2 antibody similarly detected a weak band corresponding in size to pro-PAP2 in samples with Egf1.0 (Fig. 5A). This lower level of interaction potentially reflects concentration, as PAP2 is less abundant in plasma than PAP1 and PAP3 (5–7). The ∼50-kDa band we detected using this antibody could also consist of two PAPs as PAP2 antiserum cross-reacts with PAP3 (6). PAP2 antiserum also detected an ∼11-kDa band in the Egf1.0 and control samples smaller than the expected size (∼21 kDa) of the PAP2 clip domain, suggesting this may be an unknown cross-reacting factor. Repeating these experiments using Egf1.0ΔCD and Egf1.0ΔRD confirmed that Egf1.0 binds PAP1 and PAP3 through the RD (Fig. 5B).
FIGURE 5.
Identification of PO cascade components that interact with Egf1.0. Plasma or plasma containing recombinant Egf proteins (200 pmol) was Ni-NTA column purified. Eluted proteins were then subjected to SDS-PAGE under reducing conditions and immunoblotting. A, immunoblots of plasma only (cont) compared with plasma plus wild-type Egf1.0 using PAP1, PAP2, and PAP3 antibodies. B, immunoblots of plasma only (cont) compared with plasma plus Egf1.0, Egf1.0ΔRD, or Egf1.0ΔCD using PAP1 or PAP3 antibodies. C, immunoblots of plasma only (cont) compared with plasma plus wild-type Egf1.0 using SPH1, SPH2, PO, HP14, and HP21 antibodies. Sizes of the molecular mass markers are indicated to the left of each blot.
Using antibodies to other components of the PO cascade, we detected little or no interaction between Egf1.0 and SPH1, HP14, and HP21 (Fig. 5C). However, bands corresponding to pro-SPH2 and processed SPH2 were detected in the Egf1.0 and control samples with intensity of the pro-SPH2 band being consistently stronger in the Egf1.0 sample (Fig. 5C). We detected bands corresponding to pro-PO in the Egf1.0 sample, but bands of similar intensity corresponding to pro-PO and PO were also detected in control samples, suggesting nonspecific binding. We further examined whether these interactions reflected binding to Egf1.0 by conducting immunoprecipitation experiments using an Egf1.0-specific antibody (26) and protein A beads. These experiments, however, yielded very similar results as our Ni-NTA assays. Specific binding of PAP1–3 to Egf1.0 was consistently detected, and although intensity of bands corresponding to SPH2 and pro-PO were stronger in samples containing Egf1.0, nonspecific binding was also detected in control samples without Egf1.0 (data not presented).
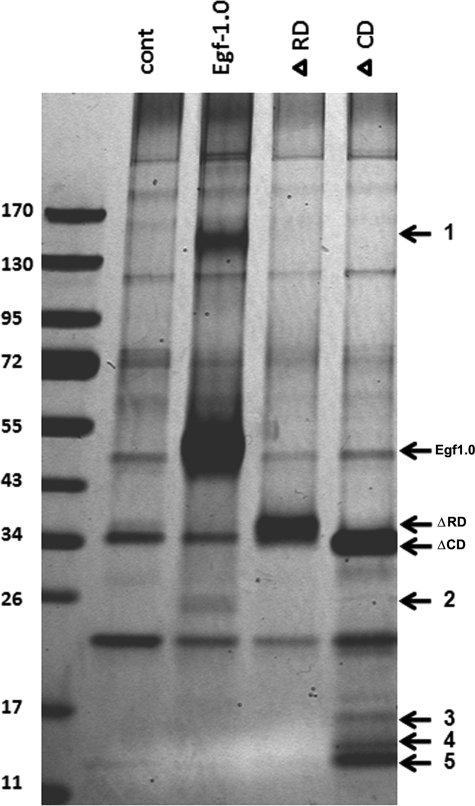
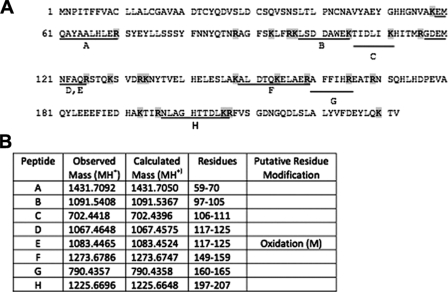
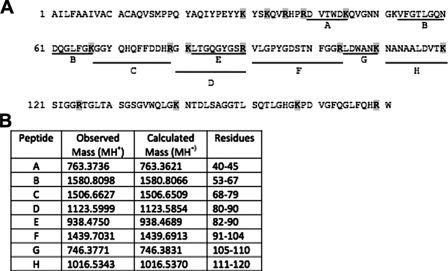
To assess whether Egf1.0 selectively bound any unknown proteins, we incubated plasma with Egf1.0, Egf1.0ΔCD, and Egf1.0ΔRD and then compared proteins eluted from Ni-NTA columns to a control sample without Egf1.0 by SDS-PAGE and silver staining. This approach cannot detect PAPs or other serine proteinases of the PO cascade because of their low abundance in M. sexta hemolymph. However, we reasoned such an approach was appropriate for detecting more abundant hemolymph proteins that potentially interact with Egf1.0. We further reasoned proteins of potential interest would more likely bind wild-type Egf1.0 and/or Egf1.0ΔRD than Egf1.0ΔCD. Our results revealed no differences in hemolymph proteins detected in the control and Egf1.0ΔRD samples, suggesting no strong binding interactions between hemolymph proteins and the CD of Egf1.0. In contrast, five bands with molecular masses of ∼140 (band 1), 26 (band 2), 17 (band 3), 14 (band 4), and 13 (band 5) kDa were detected in the Egf1.0 and/or Egf1.0ΔCD samples that were absent in the control sample (Fig. 6). We attempted to identify these components by excising the bands from gels, performing in-gel tryptic digests, and subjecting the resulting fragments to peptide mass fingerprint analysis. Masses of tryptic peptides derived from band 2 matched well with those predicted from M. sexta ferritin (Fig. 7), whereas tryptic peptides from band 5 matched those predicted for M. sexta gloverin, a type of antimicrobial peptide (Fig. 8). Peptide mass fingerprints of the other bands did not match any known protein from M. sexta or other insects.
FIGURE 6.
Identification of novel plasma proteins that interact with Egf1.0. Plasma only (cont) or plasma containing Egf1.0, Egf1.0ΔRD, or Egf1.0ΔCD (200 pmol) was Ni-NTA column-purified followed by SDS-PAGE and silver staining of eluted proteins. Five novel bands detected in the plasma plus Egf1.0 (1 and 2) or plasma plus Egf1.0ΔCD (3–5) samples that were absent in the control sample are indicated to the right of the gel. The masses of Egf1.0, Egf1.0ΔRD, or Egf1.0ΔCD are also indicated to the right of the gel, whereas the sizes of the molecular mass markers are indicated to the left.
FIGURE 7.
Identification of M. sexta ferritin in band 2 by MALDI-TOF mass spectrometry. A, amino acid sequence of mature light chain ferritin (GenBank™ accession number AAF44717). Lines below the sequence indicate the tryptic peptides that matched the masses found in the mass spectral analysis. Uppercase letters correspond to the matching masses listed in column 1 of B. B, the observed and corresponding calculated monoisotopic (MH+) masses for the tryptic peptides indicated in A are listed in columns 2 and 3. Peptides with putative residue modifications are indicated in column 4.
FIGURE 8.
Identification of M. sexta gloverin in band 5 by MALDI-TOF mass spectrometry. A, amino acid sequence of gloverin (GenBank™ number AAO74639). Lines below the sequence indicate the tryptic peptides that matched the masses found in the mass spectral analysis. Uppercase letters correspond to the matching masses listed in column 1 of B. B, observed and corresponding calculated monoisotopic (MH+) masses for the tryptic peptides are indicated as in A.
DISCUSSION
M. demolitor bracovirus encodes multiple virulence factors that together disable both cellular and humoral immune defenses of host insects (34–37). We detected Egf1.0 in host hemolymph within 2 h of infection, and expression continued over the ensuing 7 days required for M. demolitor to develop (23, 24). During this period the ability of host plasma to melanize remains suppressed, which is beneficial to survival of the wasp (26). Using M. sexta, we previously determined that Egf1.0 blocks melanization in part by competitively inhibiting the enzymatic activity of PAP3 (26). In the current study we found that Egf1.0 also inhibits the amidolytic activity of PAP1 and dose-dependently blocks processing of pro-PAP1 and pro-PAP3. Collectively, these results provide important insight into why in earlier studies we found that Egf1.0ΔCD has no effect on PAP3 amidolytic activity but still inhibits melanization of hemolymph after immune activation (26). Taken together, our results also provide strong evidence that Egf1.0 is a dual activity inhibitor that blocks both processing and the enzymatic activity of multiple PAPs. In contrast, our data reaffirm that Egf0.4 has no inhibitory activity toward the PO cascade in M. sexta. Thus, the possible target enzyme(s) and function of Egf0.4 in parasitism remains unknown. Although several insect pathogens, including multiple polydnaviruses, inhibit the melanization response of their hosts (18, 21, 38, 39), little is known about the virulence factors responsible or their mode of action. To our knowledge Egf1.0 provides the first example for how a specific, pathogen-encoded gene product suppresses the PO cascade.
That Egf1.0 inhibits multiple PAPs in M. sexta is not surprising given their similar primary structures and conserved ability to activate pro-PO at Arg51 (5, 6, 40, 41). The P2-P2′ position within the CD of Egf1.0 contains the sequence Tyr-Arg-Phe-Gln, which is similar to the cleavage site of M. sexta pro-PO (Asn-Arg-Phe-Gly) as well as that of pro-POs from other insects (5, 6, 26, 40, 41). The ability of Egf1.0 to block hemolymph melanization in several Lepidoptera as well as phylogenetically more distant insects (26) further suggests this viral protein likely inhibits PAPs from diverse species. The RD of Egf1.0 appears to be especially critical for recognition and inhibition of pro-PAP processing. On the other hand our results indicate that Egf1.0ΔCD does not block processing as well as wild-type Egf1.0. How Egf1.0 disables pro-PAP processing is currently unclear. One possibility is that binding to pro-PAP2 and pro-PAP3 interferes with HP21 that was recently shown to activate both enzymes (9, 10). However, Egf1.0 binding likely also interferes with the activity of other serine proteinases given that HP21 does not activate pro-PAP1, and other factors in hemolymph besides HP21 are able to activate pro-PAP3 (9, 10).
Consistent with its inhibitory activity toward PAPs, the presence of Egf1.0 prevents processing of pro-PO to PO in both activated plasma and pro-PO complexes formed in vitro. Somewhat surprising though is our finding that Egf1.0 also inhibits processing of pro-SPH1 and pro-SPH2 into SPH cofactors required for generating active PO (11, 42). Pro-SPH1 and pro-SPH2 processing activity has been partially purified from M. sexta plasma that contains PAP1, HP1, and small amounts of other HPs (8). One possibility then is that Egf1.0 blocks pro-SPH processing activity by inhibiting PAP1.
Our pulldown experiments using nickel beads indicate that Egf1.0 binds both pro-PAPs and PAPs through the RD and also suggest that Egf1.0 does not bind or binds only weakly other known components of the PO cascade. We did detect a somewhat higher level of SPH2 binding to nickel beads in the presence of Egf1.0 compared with control beads without Egf1.0. We speculate that SPH2 may weakly interact with Egf1.0 through its non-covalent interactions with PAPs given that complexes comprised of SPHs, pro-PO, and PAPs form in association with pattern recognition proteins after binding microbial polysaccharides (11, 43).
While recognizing the limitations of our Ni-NTA approach to identifying possible unknown proteins that might interact with Egf1.0, we felt such experiments were important to conduct because of the complexity of the PO cascade in insects and possibility that other hemolymph proteins of potential importance in immunity may interact with Egf proteins. Our results suggest that Egf1.0 binds both ferritin and the antimicrobial peptide gloverin which have not previously been identified as components of the PO cascade but that are well known immune proteins in insects (44–47). Proteomic analyses in Drosophila implicate ferritin and pro-PO in clotting (48), whereas in mammals the relative abundance of ferritin versus free iron has been suggested to play a role in regulating tyrosinase activity and the abundance of cytotoxic, reactive intermediates during formation of melanin (49, 50). Future studies, however, will be required to assess whether interactions between Egf1.0 and ferritin or gloverin are functionally important for immune suppression.
Evidence from M. sexta and other insects including Drosophila and the mosquito An. gambiae suggest the PO cascade is branched with different foreign intruders activating different arms of the cascade that later converge to activate pro-PAPs and pro-PO (9, 14, 15). Activation of different branches of the PO cascade seems especially likely in the case of metazoan parasites like parasitoid wasps and nematodes that rely on microbial symbionts with different surface features from themselves for successful infection of their insect hosts (20, 21). Targeting downstream components of the PO cascade like PAPs or pro-PO is, thus, one strategy by which polydnaviruses could broadly suppress melanization triggered by different elicitors. A dual activity inhibitor like Egf1.0 may also be advantageous in expanding the host range of parasitoids like M. demolitor that are known to parasitize multiple insect species.
Acknowledgments
We thank R. Suderman and K. D. Clark for suggestions during the course of the study, M. R. Kanost for providing the serpin 3 antiserum and useful input, and X.-Q. Yu for providing the SPH1 and SPH2 antisera. Mass fingerprint analyses were performed by the Nevada Proteomics Center, which is supported by National Institutes of Health Grant P20 RR-016464 from the Idea Network of Biomedical Research Program of the National Center for Research Resources.
This work was supported, in whole or in part, by National Institutes of Health Grant and GM58634 (to H. J.). Support for the work was also derived from United States Department of Agriculture NRI Grants 2005-05382 and 2007-04944 and the Georgia Agricultural Experiment Station (to M. R. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PO, phenoloxidase; CD, cysteine-rich domain; HP14, hemolymph proteinase 14; HP21, hemolymph proteinase 21; IEARpNA, IEAR-p-nitroanilide; PAP, prophenoloxidase activating proteinase; RD, Egf family repeat domain; smapin, small serine proteinase inhibitor; SPH, serine proteinase homolog; SPN, serpin; Pipes, 1,4-piperazinediethanesulfonic acid; Ni-NTA, nickel-nitrilotriacetic acid; MALDI, matrix-assisted laser desorption ionization; TOF, time-of-flight.
References
- 1.Cerenius, L., and Soderhall, K. (2004) Immunol. Rev. 198 116–126 [DOI] [PubMed] [Google Scholar]
- 2.Kanost, M. R., and Gorman, M. J. (2008) in Insect Immunity (Beckage, N. E., ed) pp. 69–96, Academic Press, Inc., San Diego, CA
- 3.Ji, C., Wang, Y., Ross, J., and Jiang, H. (2003) Protein Expression Purif. 29 235–243 [DOI] [PubMed] [Google Scholar]
- 4.Wang, Y., and Jiang, H. (2006) J. Biol. Chem. 281 9271–9278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang, H., Wang, Y., and Kanost, M. R. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 12220–12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang, H., Wang, Y., Yu, X.-Q., Zhu, Y., and Kanost, M. R. (2003) Insect Biochem. Mol. Biol. 33 1049–1060 [DOI] [PubMed] [Google Scholar]
- 7.Jiang, H., Wang, Y., Yu, X.-Q., and Kanost, M. R. (2003) J. Biol. Chem. 278 3552–3561 [DOI] [PubMed] [Google Scholar]
- 8.Lu, Z., and Jiang, H. (2008) Insect Biochem. Mol. Biol. 38 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorman, M. J., Wang, Y., Jiang, H., and Kanost, M. R. (2007) J. Biol. Chem. 282 11742–11749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang, Y., and Jiang, H. (2007) Insect Biochem. Mol. Biol. 37 1015–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu, X.-Q., Jiang, H., Wang, Y., and Kanost, M. R. (2003) Insect Biochem. Mol. Biol. 33 197–208 [DOI] [PubMed] [Google Scholar]
- 12.Lee, S. Y., Cho, M. Y., Hyun, J. H., Lee, K. M., Homma, K. I., Natori, S., Kawabata, S. I., Iwanaga, S., and Lee, B. L. (1998) Eur. J. Biochem. 257 615–621 [DOI] [PubMed] [Google Scholar]
- 13.Satoh, D., Horii, A., Ochiai, M., and Ashida, M. (1999) J. Biol. Chem. 274 7441–7453 [DOI] [PubMed] [Google Scholar]
- 14.Volz, J., Muller, H. M., Zdanowicz, A., Kafatos, F. C., and Osta, M. A. (2006) Cellular Microbiol. 8 1392–1405 [DOI] [PubMed] [Google Scholar]
- 15.Tang, H., Kambris, Z., Lemaitre, B., and Hashimoto, C. (2006) J. Biol. Chem. 281 28097–28104 [DOI] [PubMed] [Google Scholar]
- 16.Paskewitz, S. M., Andreev, O., and Shi, L. (2006) Insect Biochem. Mol. Biol. 36 701–711 [DOI] [PubMed] [Google Scholar]
- 17.Leclerc, V., Perte, N., El Chamy, L., Martinelli, C., Ligoxygakis, P., Hoffmann, J. A., and Reichart, J. M. (2006) EMBO Rep. 7 231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strand, M. R., and Pech, L. L. (1995) Annu. Rev. Entomol. 40 31–56 [DOI] [PubMed] [Google Scholar]
- 19.Schmidt, O., Theopold, U., and Strand, M. (2001) BioEssays 23 344–351 [DOI] [PubMed] [Google Scholar]
- 20.Goodrich-Blair, H., and Clarke, D. J. (2007) Mol. Microbial. 64 260–268 [DOI] [PubMed] [Google Scholar]
- 21.Webb, B. A., and Strand, M. R. (2005) in Comprehensive Molecular Insect Science (Gilbert, L. I., Iatrou, and Gill, S. S., eds) pp. 323–360, Vol. 6, Elsevier, San Diego, CA [Google Scholar]
- 22.Pennacchio, F., and Strand, M. R. (2006) Annu. Rev. Entomol. 51 233–258 [DOI] [PubMed] [Google Scholar]
- 23.Strand, M. R., Witherell, S. A., and Trudeau, D. (1997) J. Virol. 71 2146–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trudeau, D., Witherell, A. R., and Strand, M. R. (2000) J. Gen. Virol. 81 3049–3058 [DOI] [PubMed] [Google Scholar]
- 25.Webb, B. A., Strand, M. R., Deborde, S. E., Beck, M., Hilgarth, R. S., Kadash, K., Kroemer, J. A., Lindstrom, K. G., Rattanadechakul, W., Shelby, K. S., Thoetkiattikul, L., Turnbull, M. W., Barney, W. E., and Witherell, W. E. (2006) Virology 347 160–174 [DOI] [PubMed] [Google Scholar]
- 26.Beck, M. H., and Strand, M. R. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 19267–19272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zang, X., and Maizels, R. M. (2001) Trends Biochem. Sci. 26 191–197 [DOI] [PubMed] [Google Scholar]
- 28.Gupta, S, Wang, Y., and Jiang, H. (2005) Protein Expression Purif. 39 261–268 [DOI] [PubMed] [Google Scholar]
- 29.Wang, Y. and Jiang, H. (2004) Insect Biochem. Mol. Biol. 34 731–742 [DOI] [PubMed] [Google Scholar]
- 30.Jiang, H., Wang, Y., Ma, C., and Kanost, M. R. (1997) Insect Biochem. Mol. Biol. 27 835–850 [DOI] [PubMed] [Google Scholar]
- 31.Mortz, E., Krogh, T. N., Vorum, H., and Gorg, A. (2001) Proteomics 11 1359–1363 [DOI] [PubMed] [Google Scholar]
- 32.Rosenfeld, J., Capdevielle, J., Guillemot, J. C., and Ferrara, P. (1992) Anal. Biochem. 203 173–179 [DOI] [PubMed] [Google Scholar]
- 33.Zhu, Y., Wang, Y., Gorman, M. J., Jiang, H., and Kanost, M. R. (2003) J. Biol. Chem. 278 46556–46564 [DOI] [PubMed] [Google Scholar]
- 34.Beck, M., and Strand, M. R. (2003) Virology 314 521–535 [DOI] [PubMed] [Google Scholar]
- 35.Beck, M. H., and Strand, M. R. (2005) J. Virol. 79 1861–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thoetkiattikul, L., Beck, M. H., and Strand, M. R. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 11426–11431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pruijssers, A., and Strand, M. R. (2007) J. Virol. 81 1209–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, G., Lu, Z.-Q., Jiang, H., and Asgari, S. (2004) Insect Biochem. Mol. Biol. 34 477–483 [DOI] [PubMed] [Google Scholar]
- 39.Eleftherianos, I., Boundy, S., Joyce, S. A., Aslam, S., Marshall, J. W., Cox, R. J., Simpson, T. J., Clarke, D. J., ffrench-Constrant, R. H., and Reynolds, S. E. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 2419–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall, M., Scott, T., Sugumaran, M., Soderhall, K., and Law, J. H. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 7764–7768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park, D. S., Shin, S. W., Hong, S. D., and Park, H. Y. (2000) Mol. Cell 10 186–192 [DOI] [PubMed] [Google Scholar]
- 42.Kwon, T. H., Kim, M. S., Choi, H. W., Joo, C. H., Cho, M. Y., and Lee, B. L. (2000) Eur. J. Biochem. 267 6188–6196 [DOI] [PubMed] [Google Scholar]
- 43.Tong, Y., Jiang, H., and Kanost, M. R. (2005) J. Biol. Chem. 280 14932–14942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lundstrom, A., Liu, G., Berzins, K., and Steiner, H. (2002) Insect Biochem. Mol. Biol. 32 795–801 [DOI] [PubMed] [Google Scholar]
- 45.Paskewitz, S. M., and Shi, L. (2005) Insect Biochem. Mol. Biol. 35 815–824 [DOI] [PubMed] [Google Scholar]
- 46.Lavine, M. D., Chen, G., and Strand, M. R. (2005) Insect Biochem. Mol. Biol. 35 1335–1346 [DOI] [PubMed] [Google Scholar]
- 47.Altincicek, B., Knorr, E., and Vilcinskas, A. (2008) Dev. Comp. Immunol. 32 585–595 [DOI] [PubMed] [Google Scholar]
- 48.Karlsson, C., Korayem, A. M., Scherfer, C., Loseva, O., Dushay, M. S., and Theopold, U. (2004) J. Biol. Chem. 279 52033–52041 [DOI] [PubMed] [Google Scholar]
- 49.Gotz, M. E., Double, K., Gerlach, M., Youdim, M. B., and Riederer, P. (2004) Ann. N. Y. Acad. Sci. 1012 193–208 [DOI] [PubMed] [Google Scholar]
- 50.Maresca, V., Flori, E., Cardinali, G., Briganti, S., Lombardi, D., Mileo, A. M., Paggi, M. G., and Picardo, M. (2006) J. Cell. Physiol. 206 843–848 [DOI] [PubMed] [Google Scholar]